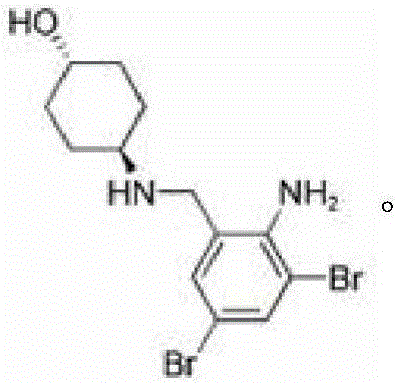
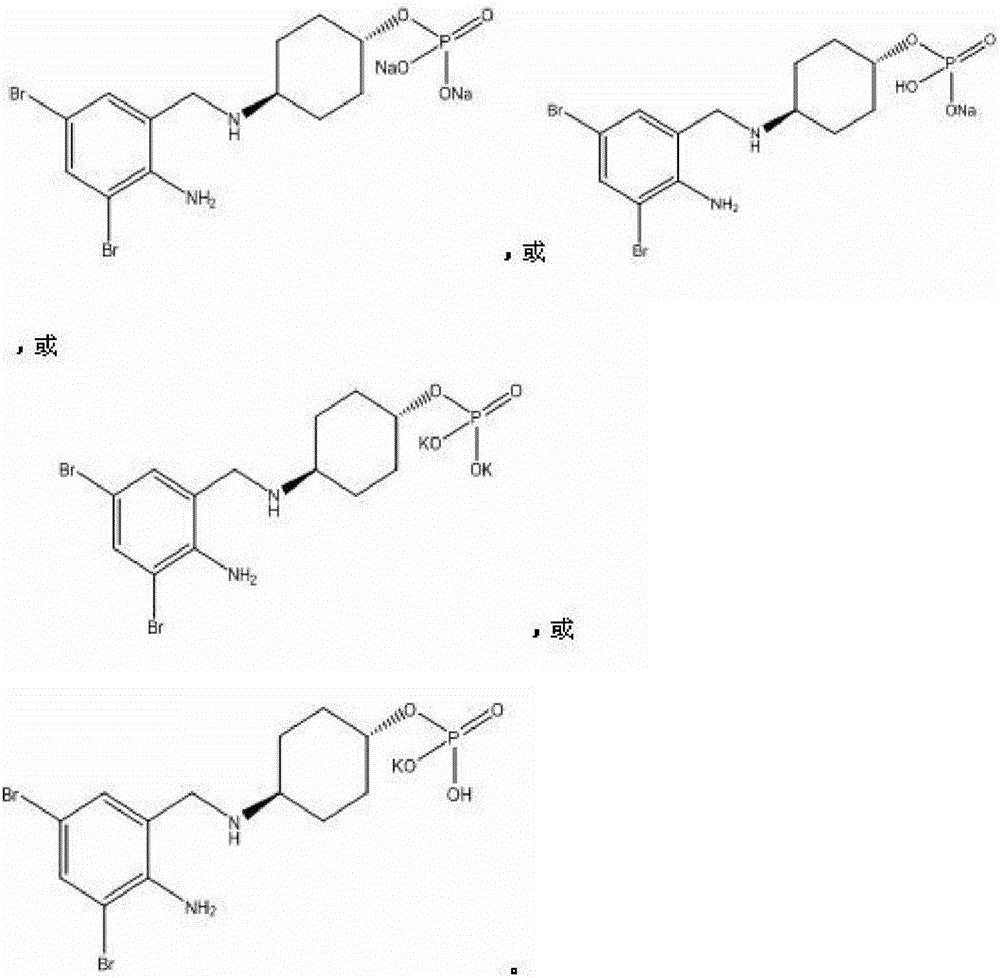
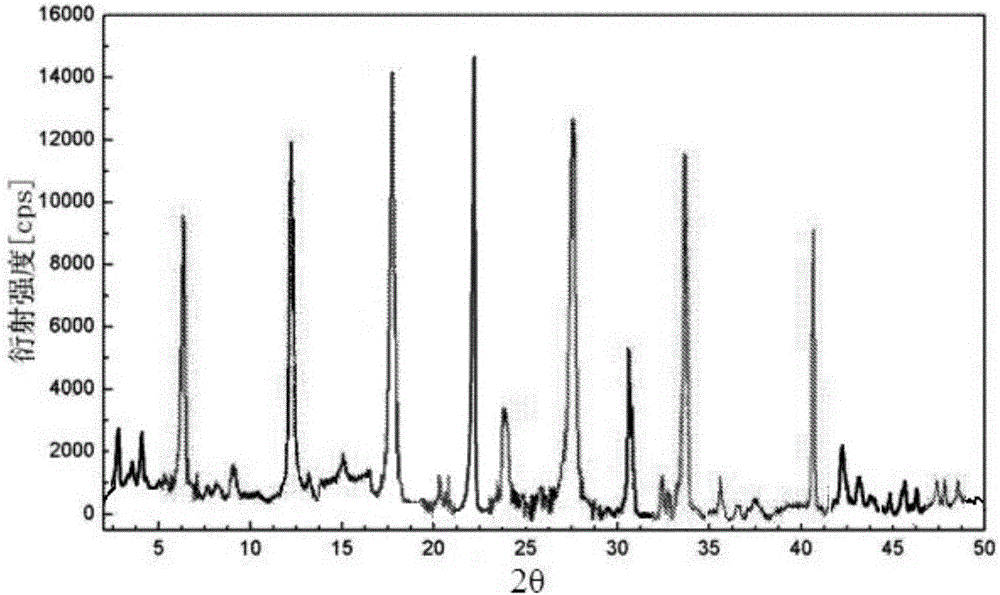
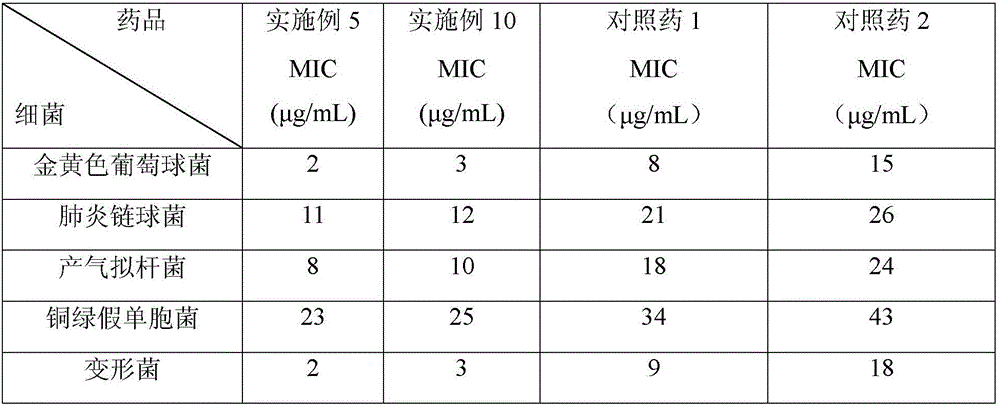
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112 results about "Cefoperazone Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
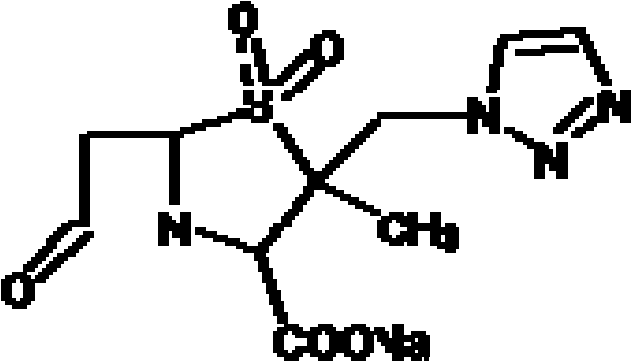
The sodium salt form of cefoperazone and a semi-synthetic, broad-spectrum, beta-lactamase resistant, third-generation cephalosporin antibiotic with bactericidal activity. Cefoperazone sodium inhibits bacterial cell wall synthesis by inactivating penicillin binding proteins (PBPs) thereby interfering with the final transpeptidation step required for cross-linking of peptidoglycan units which are a component of bacterial cell walls. This results in a reduction of cell wall stability and causes cell lysis.
High-purity medicament and preparation thereof
InactiveCN101348493AHigh purityNo pollution in the processAntibacterial agentsPowder deliveryDrug compoundTAZOBACTAM SODIUM
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for crystallizing cefoperazone sodium
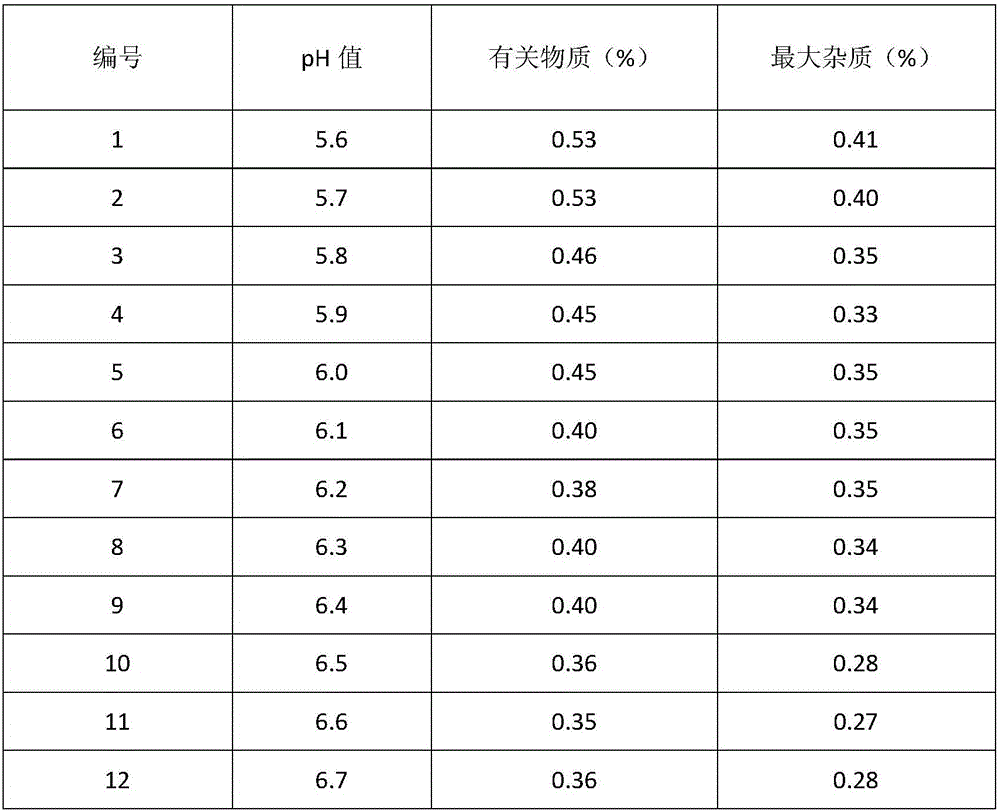
The invention aims to provide a method for crystallizing cefoperazone sodium, which comprises the following steps of: dissolving cefobid in a solvent, and adding a salt forming agent to adjust the pH to between 6.2 and 6.8 until the solid is completely dissolved; and filtering, adding the filtrate into the solvent under the ultrasonic condition to separate out crystals, filtering, washing and leaching the crystals, and performing vacuum drying to obtain the cefoperazone sodium. Aiming at the problems in the industrial crystallization of the cefoperazone sodium, the ultrasonic radiation has strong orienting effect, supplements and enhances the wave action required by the formation of critical crystal nucleus, can accelerate graining process and promote the quick generation of the crystal nucleus, and achieves the effect of quickly graining without seeding by seed crystals; meanwhile, the ultrasonic in the liquid medium can make mass points in the medium obtain high acceleration, has the effect of cavitation, and can prevent the accumulation during crystal growth to make the size distribution of the crystals uniform.
Owner:FUJIAN FUKANG PHARMA
Novel method for synthesizing cefoperazone sodium compound
The invention relates to a novel preparation method for (6R,7R)-3-[[(1-methyl-1H-tetrazole-5 radial)sulphur]methyl]-7-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazine carbon amido)-2-p-hydroxyl phenyl-acetamido]-8-keto-5-thia-1-polyaza[4.2.0]octane-2-alkene-2-sodiumformate(cefoperazone sodium) shown as a formula (I). The formula (I) is shown in the specifications. The invention aims to provide a novel method for synthesizing a cefoperazone sodium compound. The method has the advantages of synthesis of TZA from 7-ACA (Acetic Acid) and 1-methyl-5-sulfydryl tetrazole, mild reaction condition, easiness for operating, one-step use of recyclable dimethyl carbonate serving as an environmentally-friendly solvent, great saving in the cost, high yields of products prepared in each step, good quality, low cost, high product purity and suitability for industrial production.
Owner:哈药集团股份有限公司 +1
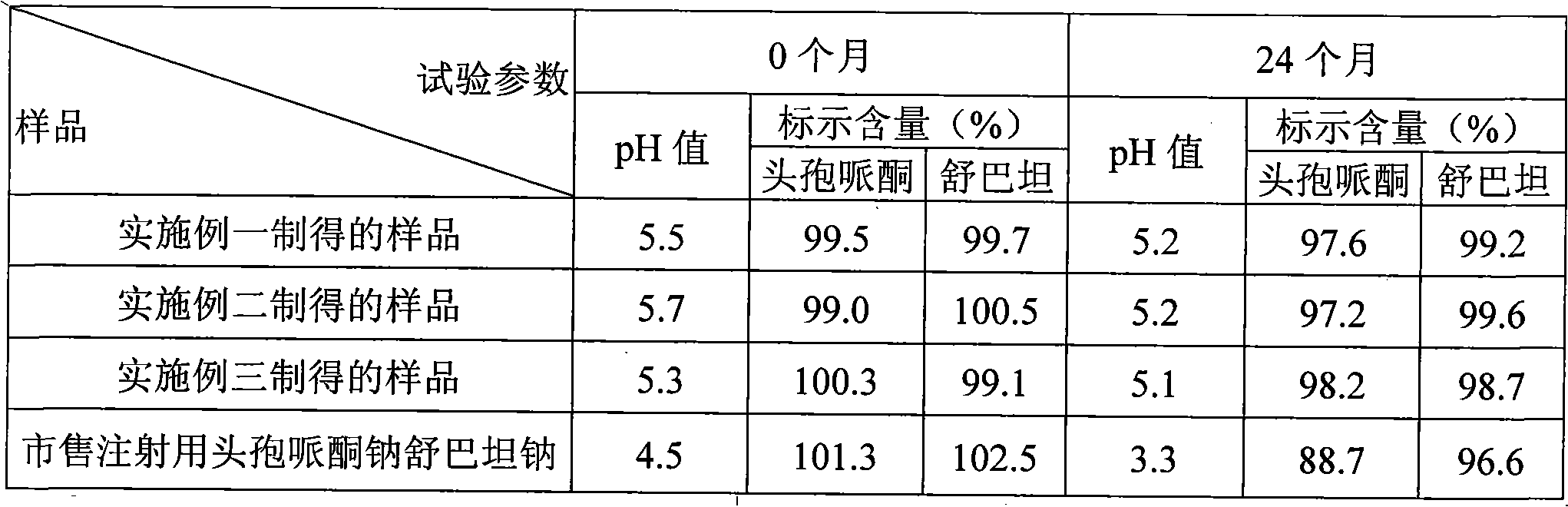
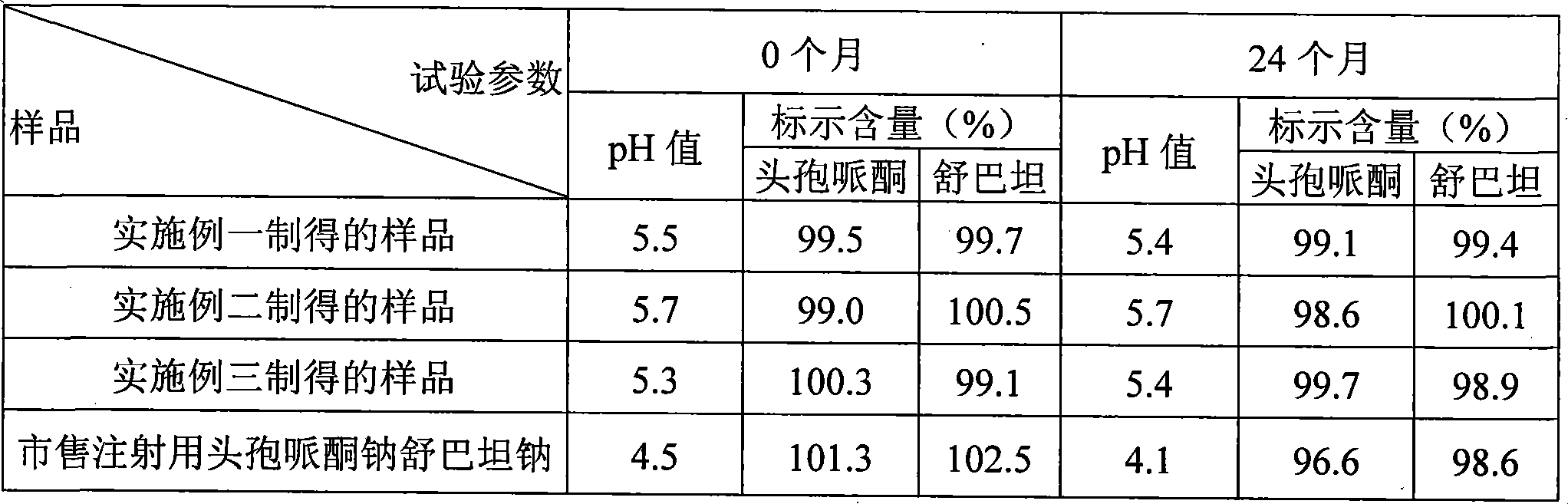
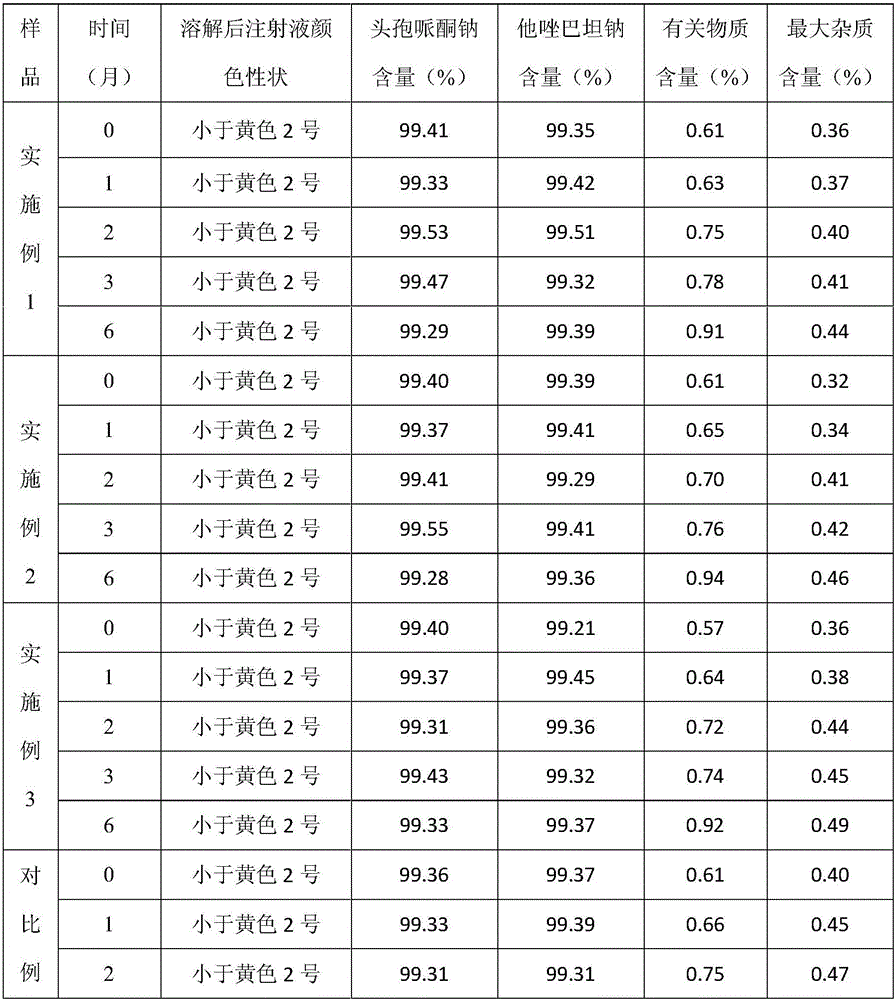
Preparation method of cefoperazone sodium and sulbactam sodium powder injection for injection
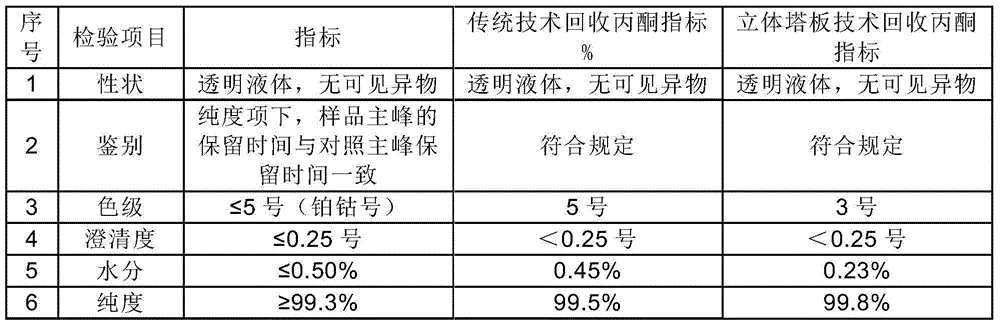
The invention discloses a preparation method of a cefoperazone sodium and sulbactam sodium powder injection for injection. A recovered solvent is prepared by a stereo mass transfer tower plate technology, and is directly applied to production of cefoperazone sodium and sulbactam sodium products; the quality indexes such as color grade, clarity and purity of the obtained cefoperazone sodium and sulbactam sodium powder injection product for injection are greatly improved; the cefoperazone sodium and sulbactam sodium powder injection is high in quality stability and few in impurity; and the preparation method is low in production cost and simple in process.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Suspension powder injection of cefoperazone sodium and tazobactam sodium pharmaceutical composition and new application thereof
InactiveCN101632677AUnexpected effectImprove stabilityPowder deliveryUrinary disorderFreeze-dryingBULK ACTIVE INGREDIENT
The invention discloses a suspension powder injection with a cefoperazone sodium and tazobactam sodium pharmaceutical composition as an active ingredient, and comprises the following components: 4 parts of the cefoperazone sodium, 1 part of the tazobactam sodium, 5-30 parts of an emulsifier, 1-15 parts of an auxiliary emulsifier and 1-40 parts of a freeze-drying support agent. The invention further discloses an application thereof in preparing medicines for treating cystitis.
Owner:HAINAN YONGTIAN PHARMA INST
Antibiotics composition with stable content and rapid solubility
ActiveCN101264088ARegulation stabilityStable madeAntibacterial agentsPowder deliveryAntibiotic YDissolution
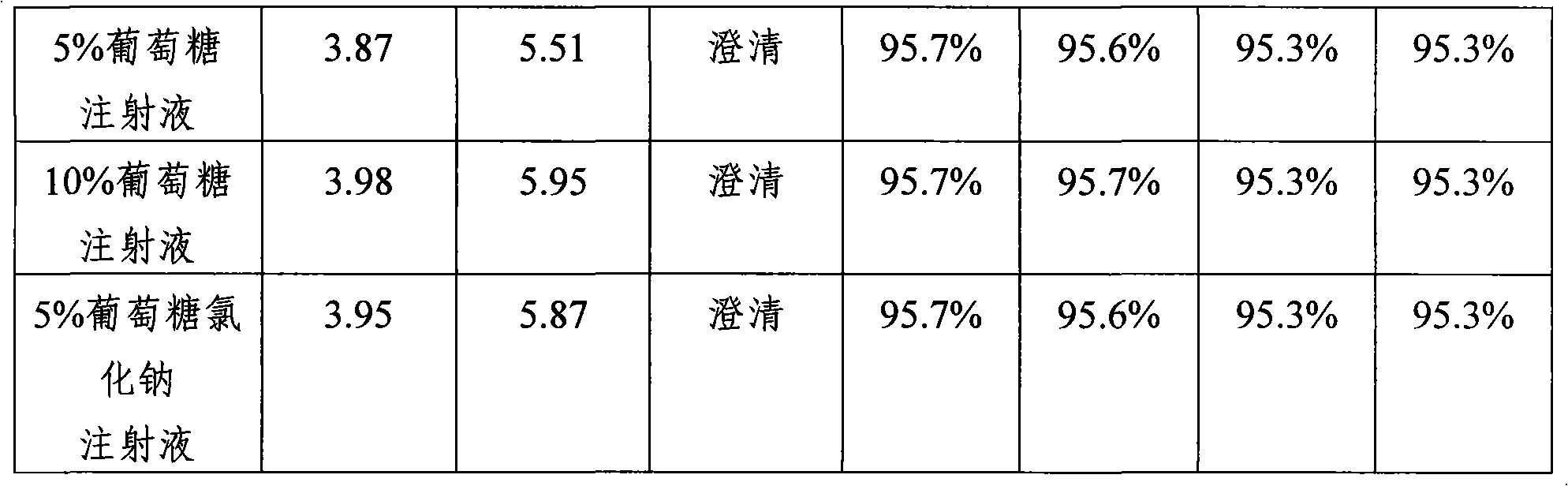
The invention discloses an antibiotics combination with stable content and rapid dissolution and the preparation method, which comprises cefoperazone sodium, Beta- lactamase inhibitor and pH regulator with weight proportion of 1 to 100 : 1 : 0.001 to 1. When the antibiotics combination is dilutedly administrated with clinical conventional infusion according to certain proportion, wherein, the content of the cefoperazone sodium can be kept stable and the dissolution is rapid without crystallization and degradation product and without influence of temperature, thereby solving the problems of content lowering of cefoperazone sodium and low solubility when similar drugs are used clinically. The antibiotics combination has the advantages of simple preparation method and high efficiency and easy industrialization.
Owner:南京丰恺思药物研发有限公司
Umbilical cord preserving fluid and preparation method thereof
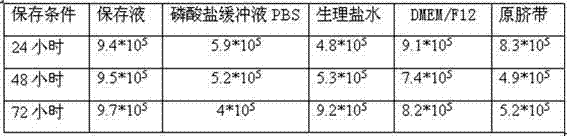
ActiveCN102334472ALower metabolismPreserve activityDead animal preservationUmbilical cord tissuePotassium
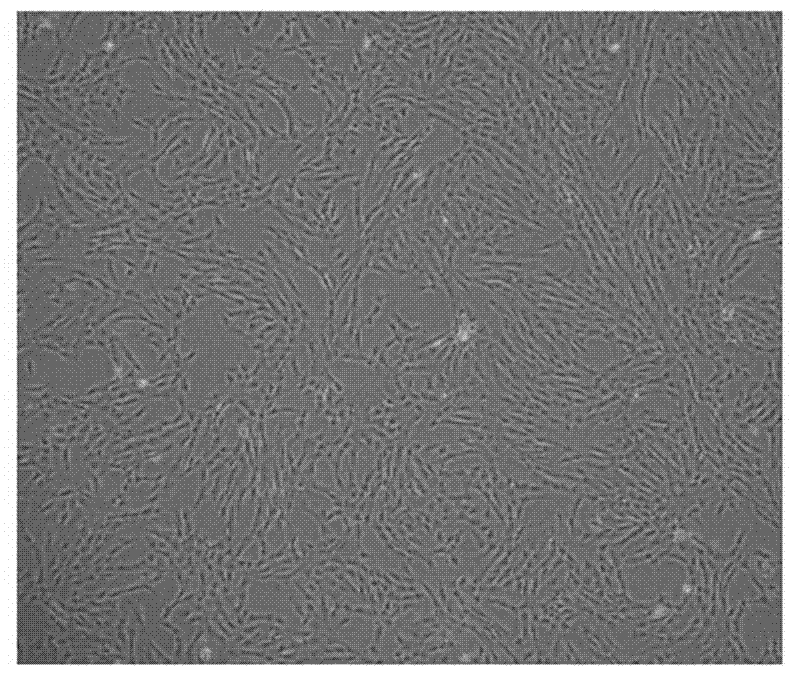
The invention discloses an umbilical cord preserving fluid for temporary preservation of umbilical cord after collection before separation. The umbilical cord preserving fluid comprises the following main components: sodium chloride, glucose, human serum albumin, potassium chloride and anhydrous calcium chloride, as well as auxiliary components: cefoperazone sodium and phenol red, wherein the main components have the effects on maintaining osmotic equilibrium of inner cells of the umbilical cord tissue, keeping the umbilical cord in a wet state and providing nutrient content for a short period of time; and the auxiliary components have the effects on preventing probable pollution and prompting happened pollution. The preserving fluid can be used for preserving the activity of mesenchyme stem cells in the umbilical cord, the umbilical cord can be effectively preserved at 2-10 DEG C for at least 72 hours, the activity of the separated mesenchyme stem cells is minimally influenced by time, the time limit from collection to preparation of the umbilical cord is greatly reduced, and all the used components meet a clinical use standard and are capable of effectively reducing the pollution probability.
Owner:江苏省北科生物科技有限公司
A kind of cefoperazone sodium tazobactam sodium pharmaceutical composition
ActiveCN102274233AReduce security risksFew kindsAntibacterial agentsHeterocyclic compound active ingredientsPowder diffractionSubstance content
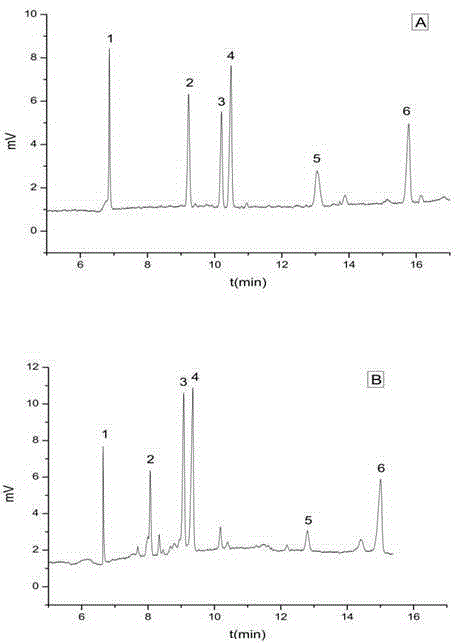
The invention relates to a medicinal composition of cefoperazone sodium and tazobactam sodium. The medicinal composition comprises the following components in part by weight: 4 to 8 parts of cefoperazone sodium and 1 part of tazobactam sodium, wherein the tazobactam sodium is measured by a powder X-ray diffraction measuring method, and characteristic diffraction peaks are shown at the positions of 6.9 degrees, 10.5 degrees, 11.4 degrees, 16.6 degrees, 19.2 degrees, 22.7 degrees, 27.0 degrees, 29.7 degrees and 33.5 degrees in an X-ray powder diffraction map expressed by a diffraction angle of between 2 theta+ / -0.2 degree. The medicinal composition has the advantages of high stability, low relevant substance content, controllable quality and the like, and the administration safety of patients is improved. The invention also relates to a method for preparing the tazobactam sodium with the technical characteristics of the characteristic diffraction peaks.
Owner:江西璟瑞药业有限公司
Method for preparing cefoperazone sodium
The invention discloses a method for preparing cefoperazone sodium. The method comprises steps as follows: dissolving: acetone and purified water are added to a reaction tank, stirring is started, cefoperazone acid is added, pH (potential of hydrogen) is adjusted to be 6.8-7.2, and cefoperazone acid is completely dissolved; decoloration: activated carbon is added to a cefoperazone acid solution for decoloration, and the cefoperazone acid solution is pressed into a crystallizing tank of a sterilizing room through a decarbonization filter and a multilevel bacterium removal filter element; crystallization: an organic solvent is fed to the crystallizing tank for crystallization; crystals grow under the stirring condition, a three-in-one filter is used for filtration, and a filter cake is washed with acetone; drying: the washed filter cake enters a drying device, sterilized and filtered methanol, water or methanol water is atomized through a direct-injection type atomization device, atomized steam is driven by sterile nitrogen to blow the filter cake for 2 h plus or minus 1.5 h, then, hot water is provided for circulation heating, the filter cake is dried in a vacuum state until residual solvents are qualified, and then cefoperazone sodium is discharged. The acetone residues in a cefoperazone sodium product are far smaller than 0.5%, the product quality and the stability are improved, medication is safer, and good social benefits are generated.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium and preparation process thereof
ActiveCN104013629AImprove solubilityImprove stabilityAntibacterial agentsRespiratory disorderSolubilityAntioxidant
The invention discloses a composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium. The composite pharmaceutical composition is an injection, and is prepared from the following components in parts by weight: 3.5-4.5 parts of cefoperazone sodium, 1 part of tazobactam sodium, 0.5-1 part of ambroxol hydrochloride, 0-6 parts of excipient, 0-10 parts of isotonic agent, 0-1 part of antioxidant, a proper amount of citric acid-sodium citrate and 20 parts of injection water. The composite preparation of the cefoperazone sodium and the tazobactam sodium disclosed by the invention is stable in quality and significant in curative effect, not only can three active ingredients be evenly mixed, but also the composite pharmaceutical composition is excellent in stability, good in solubleness and good in clinical use safety.
Owner:福安药业集团庆余堂制药有限公司
Double-template molecularly-imprinted solid-phase extraction column and application method
ActiveCN105233809AHigh enrichment efficiencyImprove purification efficiencyIon-exchange process apparatusOther chemical processesAnimal foodCapillary electrophoresis
The invention relates to the technical field of solid-phase extraction, specifically to an oxacillin-and-cephalexin double-template molecularly-imprinted solid-phase extraction column and an application method. The extraction column is cooperatively used with a capillary electrophoresis method for selective separation, enrichment and detection of residues of amoxicillin, cephalexin, oxacillin, penicillin G, cefazolin sodium and cefoperazone sodium in animal food.
Owner:JIANGSU UNIV
Cefoperazone sodium compound prepared by using fluid mechanics principle and preparation comprising cefoperazone sodium compound
InactiveCN106432273AEasy to manufactureSmall particle sizeOrganic active ingredientsOrganic chemistryMedical productX-ray
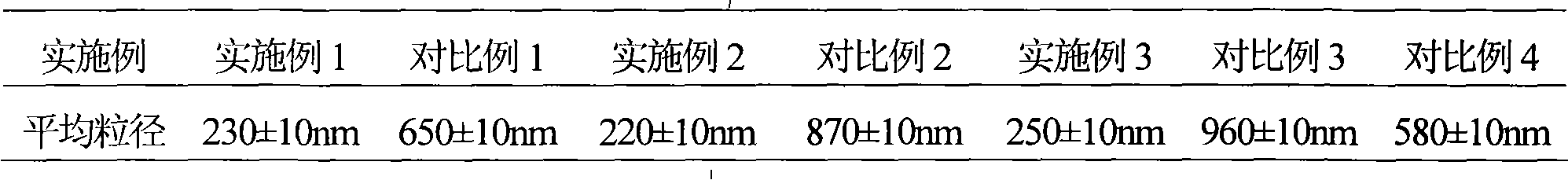
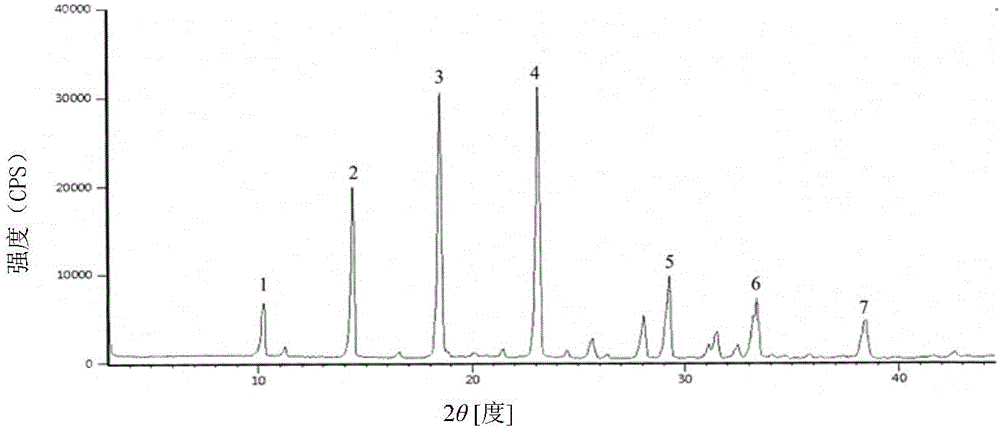
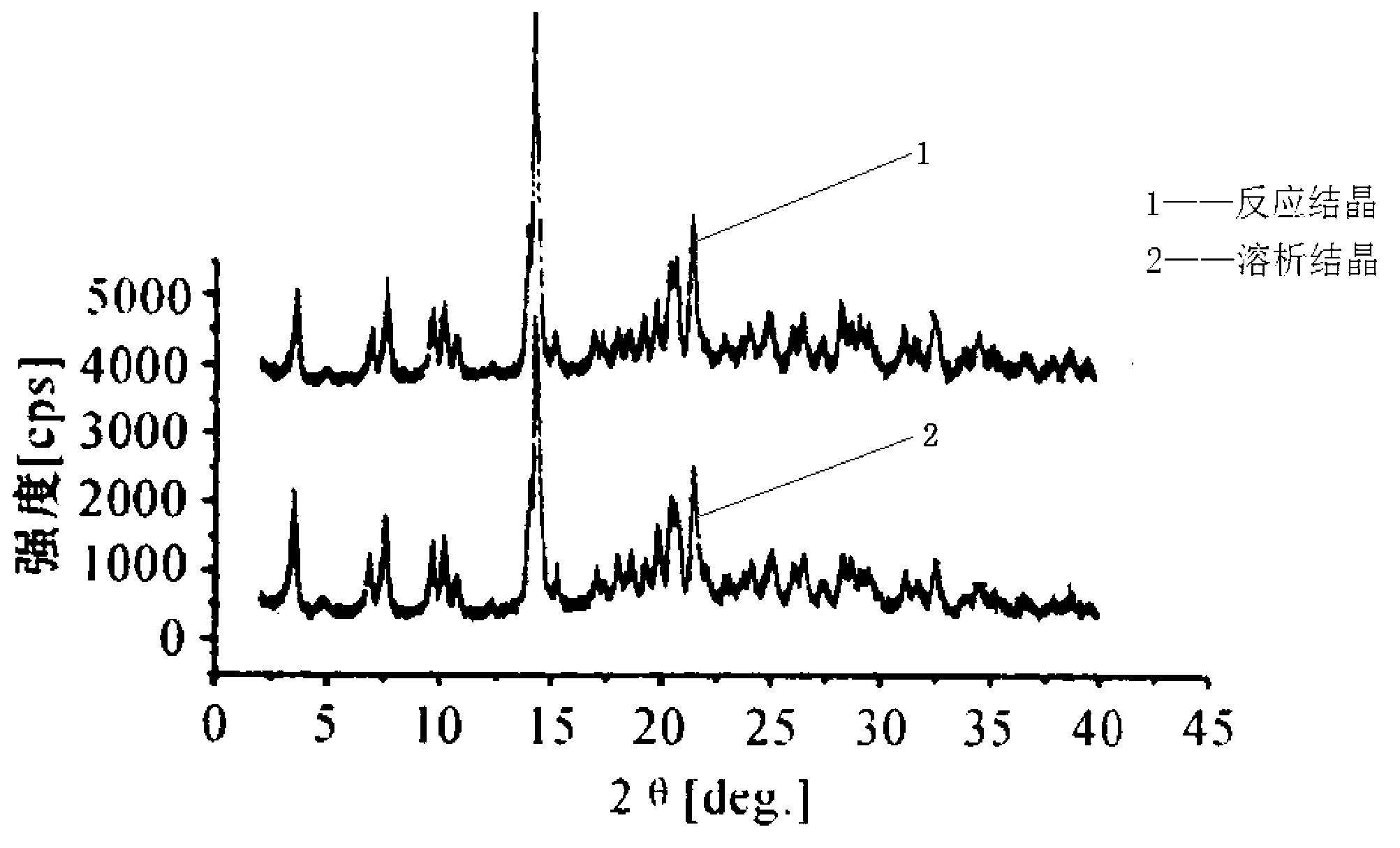
The invention discloses a cefoperazone sodium compound prepared by using the fluid mechanics principle. Research&Development and Industrialization Project of High-end Medical Product Refinement Crystallization Technologies wins the second prize of national scientific and technological progress in 2015, and the fluid mechanics principle crystallization technology belongs to one of the high-end medical product refinement crystallization technologies. The cefoperazone sodium compound is determined by using X-ray powder diffraction, and the main characteristic peaks represented by the diffraction angle 2 theta in a map are located at 10.25+ / -0.2 degrees, 14.40+ / -0.2 degrees, 18.51+ / -0.2 degrees, 23.14+ / -0.2 degrees, 29.10+ / -0.2 degrees, 33.25+ / -0.2 degrees and 38.45+ / -0.2 degrees. Cefoperazone acid reacts with a salt forming agent, and the cefoperazone sodium compound is prepared through secondary crystallization. The operation is simple, reactants are easy to obtain, the reaction condition is mild, and the yield is high. The compound is high in purity, low in impurity content, good in fluidity and good in stability. Meanwhile, the invention further discloses a preparation prepared from cefoperazone sodium, namely, cefoperazone sodium for injection. The preparation process of the preparation is simple, no excipient is needed, and the preparation has better stability and few side effects.
Owner:陕西顿斯制药有限公司
Cefoperazone sodium and sulbactam sodium combination and preparation method thereof
InactiveCN101284009AImprove stabilityGuaranteed qualifiedAntibacterial agentsPharmaceutical delivery mechanismActivated carbonFreeze-drying
The invention provides a cefoperazone sodium / sulbactam sodium composition, comprises the following components of: cefoperazone sodium, sulbactam sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride. The invention also provides a preparation method of the cefoperazone sodium / sulbactam sodium composition. The method comprises the following steps of: weighing components as prescription; dissolving the components in water, and adjusting pH to 5.0-6.5 with phosphoric acid or sodium hydroxide solution; and adsorbing with activated carbon, filtering with microporous filtering film, filling, freeze drying, subpackaging under aseptic condition, or freeze drying, pulverizing, sieving and then subpackaging under aseptic condition. The composition adds sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride in the prescription, so as to improve the stability of the compound preparation. The composition can be stored in a dark, cool and dry place, so as to ensure product quality within validity period.
Owner:管小明
Preparation of cefoperazone and sulbactam sodium mixed powder
The invention relates to a preparation method of cefoperazone sodium / sulbactam sodium mixed powder. Cefoperazone acid and sulbactam acid are prepared into water solution with 20 to 40 percent by weight according to the weight ratio of 0.9 to 2.1: 1, a salt forming agent is used for regulating the pH of material liquid to 6.0 to 6.5, a filter membrane with 0.22Mum is used for filtration, then the material liquid is arranged in a freeze-drying box; and the cefoperazone sodium / sulbactam sodium mixed powder product is obtained after freeze-drying. The product of the mixed powder obtained after freeze-drying has good uniformity, rapid dissolution speed and stable main quality indicators of product content, water content, pH value, clarification of the solution and so on.
Owner:QILU ANTIBIOTICS PHARMA
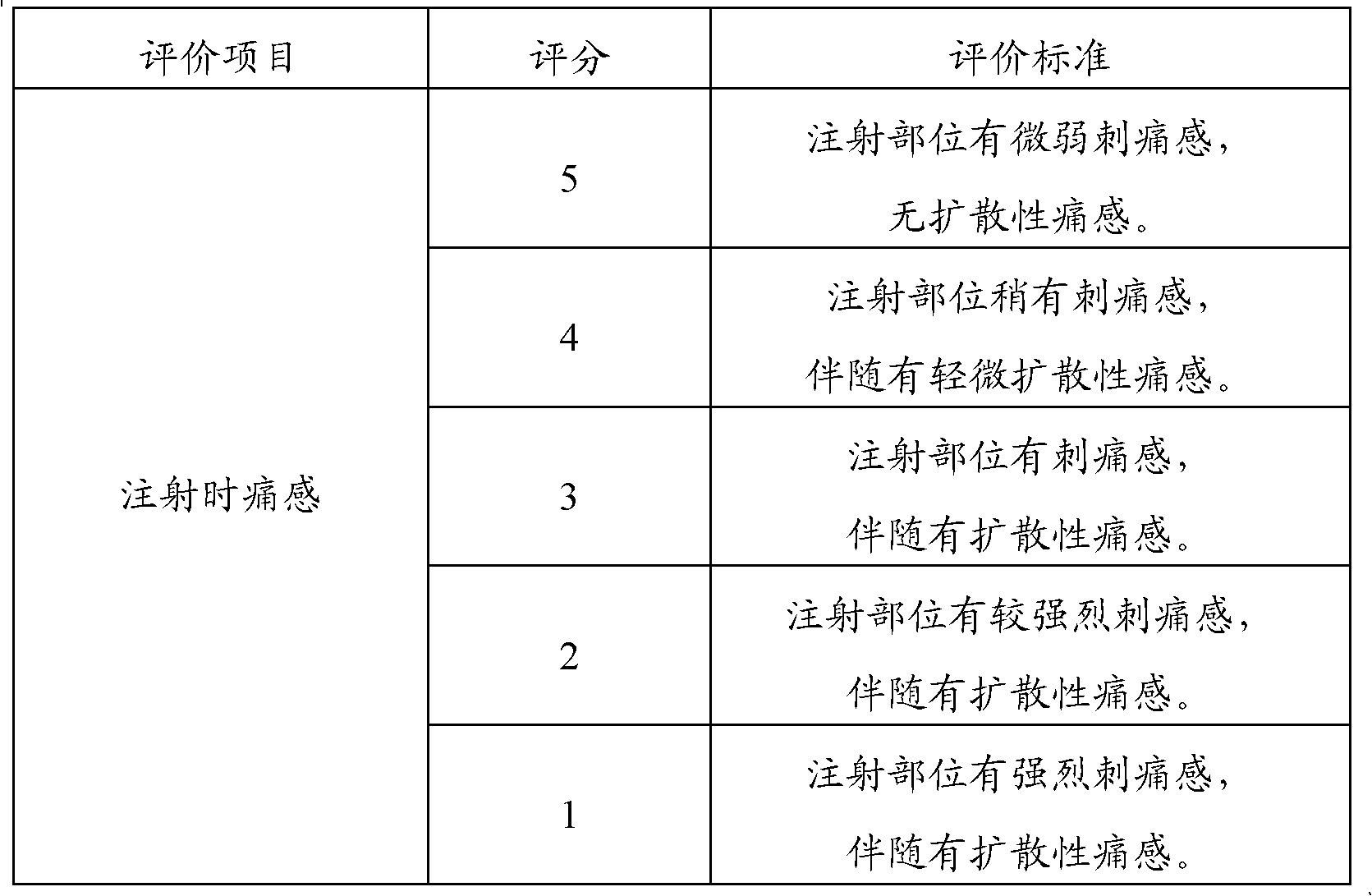
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
ActiveCN102552275AImprove efficacyAntibacterial agentsPowder deliveryCurative effectInjection powder
The invention relates to the technical field of medicine, in particular to an injection powder and injection preparation of a cefoperazone sodium-tazobactam combination. The injection powder and injection preparation comprises cefoperazone sodium, tazobactam combination and lignocaine hydrochloride, wherein the mass ratio of the cefoperazone sodium to the tazobactam combination to the lignocaine hydrochloride is 4:1:(0.01-0.05). Compared with a positive medicament control group, the injection powder and injection preparation of the cefoperazone sodium-tazobactam combination provided by the invention has the advantages of capability of relieving injection pain and remarkable improvement on the curative effect.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Method for recovering acetonitrile from waste liquor obtained in producing cefoperazone sodium
InactiveCN1660787AReduce pollutionShort processCarboxylic acid nitrile purification/separationSalting outPhysical chemistry
A process for recovering acetonitrile for the sewage generated in preparing cefoperazone sodium includes rectifying to obtain coarse acetonitrile, adding composite salting-out agent, stirring, filter, and rectifying again to obtain high-purity acetonitrile.
Owner:HARBIN INST OF TECH
Preparation method for injection cefoperazone sodium tazobactam sodium composition
InactiveCN103120692AGuaranteed uniformityAntibacterial agentsPowder deliveryCefbuperazoneTAZOBACTAM SODIUM
The invention provides a preparation method for injection cefoperazone sodium tazobactam sodium composition. The preparation method comprises the following steps of: crushing the cefoperazone sodium and the tazobactam sodium; and mixing the cefoperazone sodium and the tazobactam sodium in a weight ratio of 4:1; and separately packaging, pressing and capping the mixture. The preparation method is characterized in that the fineness of pulverization of the cefoperazone sodium and the tazobactam sodium is 50-120 meshes.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Preparation process for cefoperazone sodium and tazobactam sodium for injection
A preparation process for cefoperazone sodium and tazobactam sodium for injection is disclosed, which comprises the following steps of: (1) mixing for cefoperazone sodium powder and tazobactam sodium powder; (2) cleaning for bottle: straightening a bottle, cleaning the bottle by an ultrasonic wave, and sterilizing by dry heat in a tunnel drying oven; (3) cleaning for stopper: cleaning a rubber stopper, sterilizing and drying; (4) sterile subpackaging; (5) treatment for aluminium cap; (6) lamp inspection for capping; (7) labelling; and (8) boxing.
Owner:SUZHOU ERYE PHARMA CO LTD
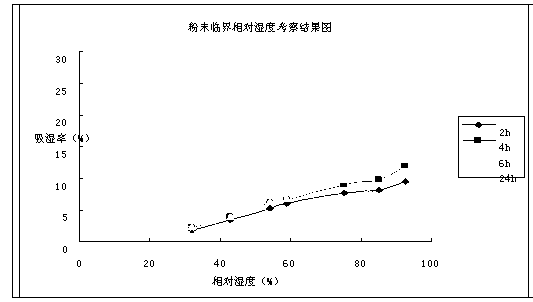
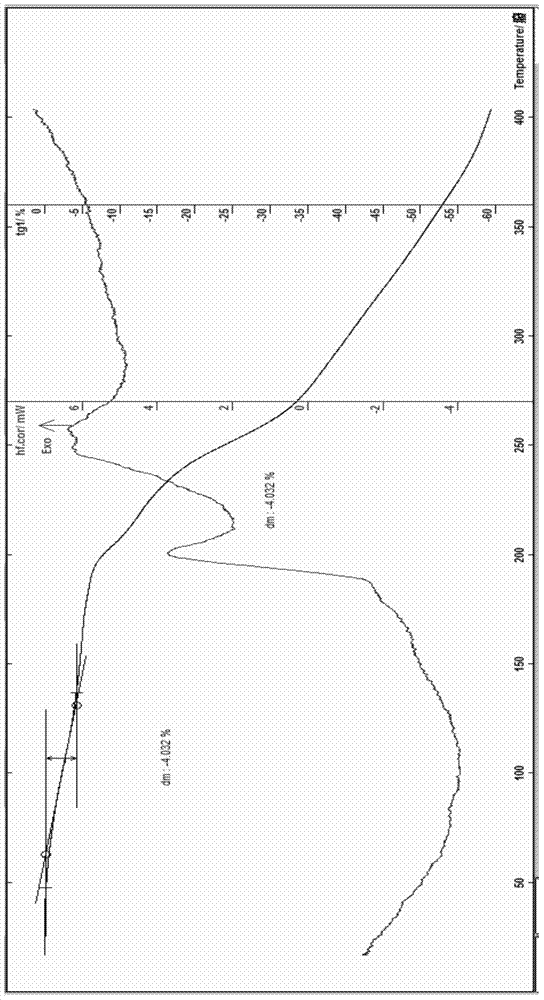
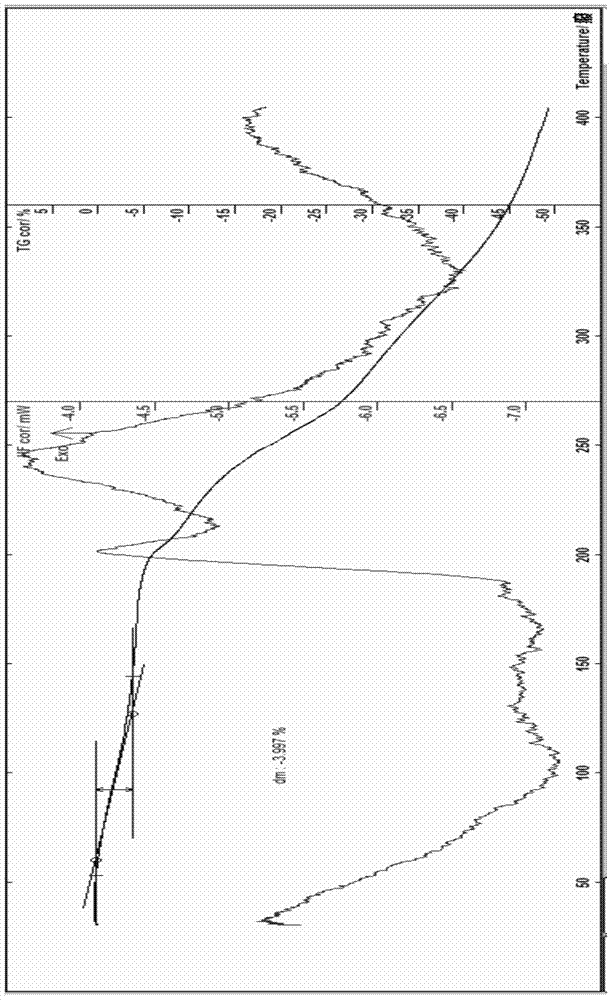
Cefoperazone sodium compound entity, composition and application
InactiveCN104327099AReduce humidityEasy to makeAntibacterial agentsOrganic chemistry methodsBacteroidesBacilli
The invention provides a cefoperazone sodium compound entity and a composition thereof. The cefoperazone sodium compound entity is low in hygroscopicity and good in storage stability, is suitable in an application of preparing drugs for treating or preventing respiratory system infection, infection in the five sense organs, urinary system infection, pelvic cavity infection, septicemia, skin soft tissue infection, infection in bones and joints, gonorrhea and meningitis of human or animal due to bacteria being sensitive to gram-positive bacteria or gram-negative bacteria.
Owner:LIANHE KANGXING BEIJING PHARMA
Novel cefoperazone sodium and sulbactam sodium pharmaceutical composition for injection
InactiveCN106309448ASimple stepsEasy to operateAntibacterial agentsPowder deliverySulbactam SodiumChloride sodium
The invention discloses a novel cefoperazone sodium and sulbactam sodium pharmaceutical composition for injection, including the following components: 23o-24o of sulbactam sodium, sodium cefoperazone of specific rotation, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride, of where the weight weight-ratio of sulbactam sodium, cefoperazone sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride is 1:1-4:0.25-0.30:0.16-0.20:0.11-0.15. The invention also discloses a preparing method of the described new cefoperazone sodium and sulbactam sodium pharmaceutical composition for injection. The novel cefoperazone sodium and sulbactam sodium pharmaceutical composition has good stability, which can ensure qualified products within date expiration leads to clinically safe medication use.
Owner:南昌立健药业有限公司
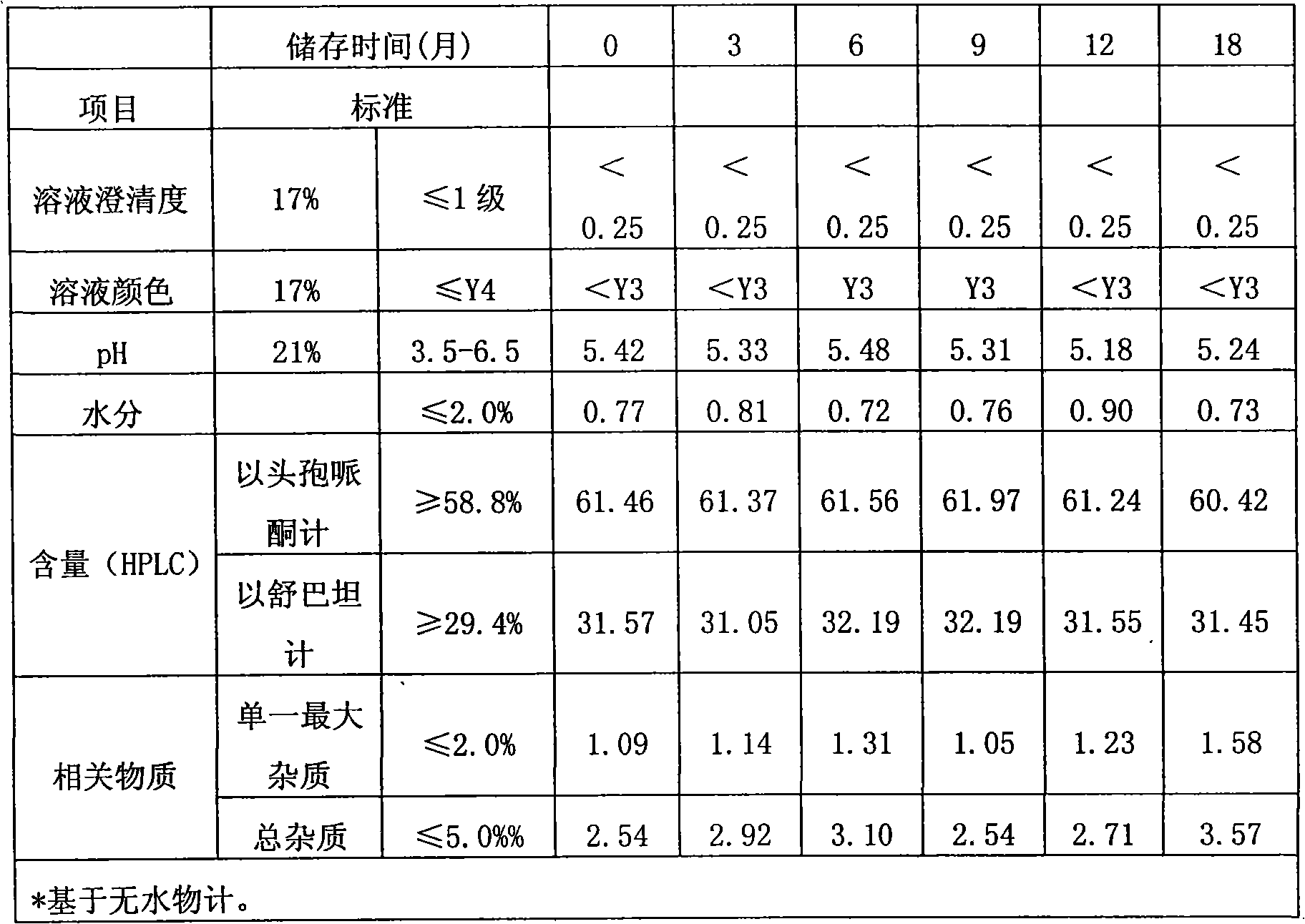
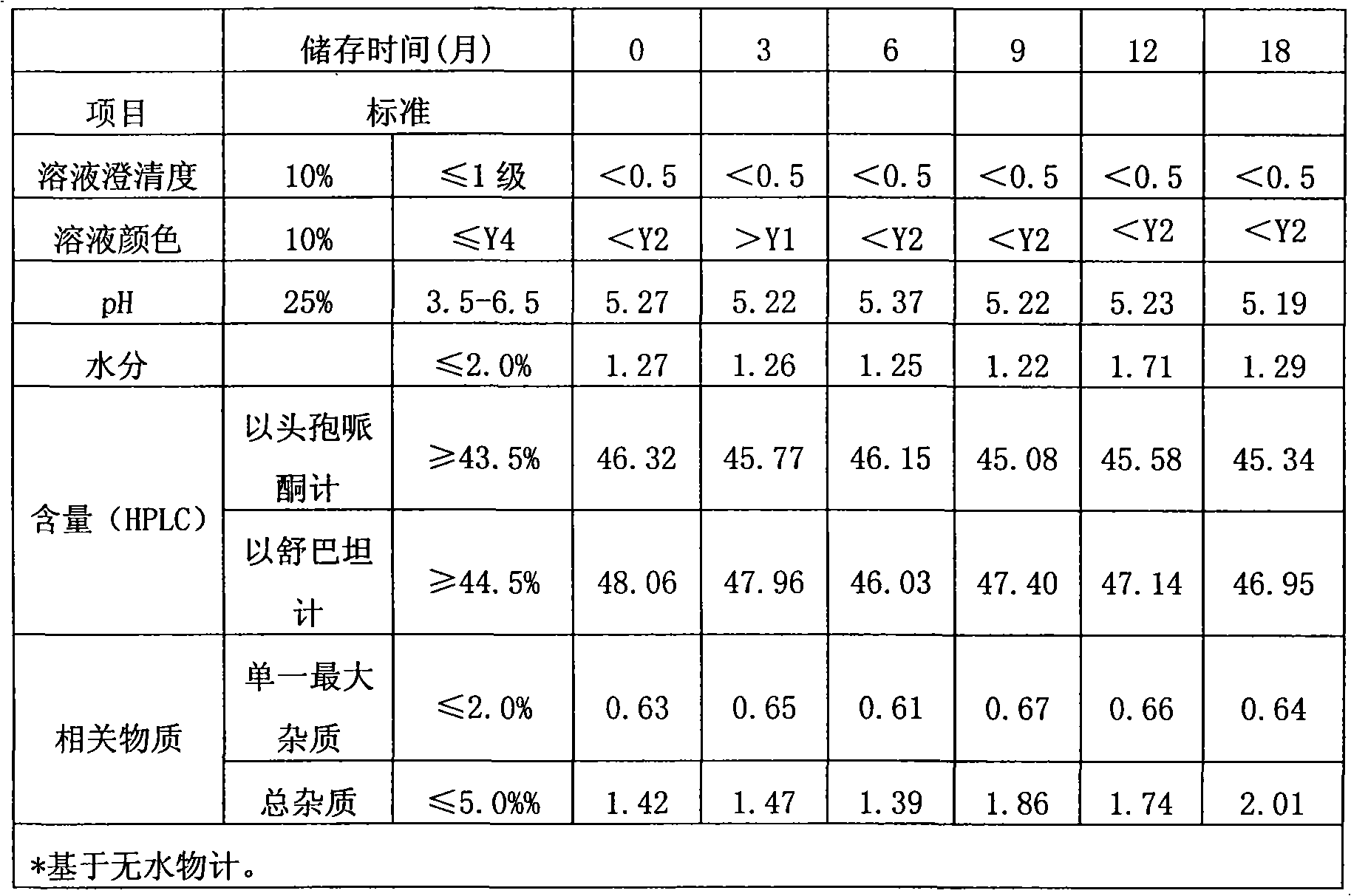
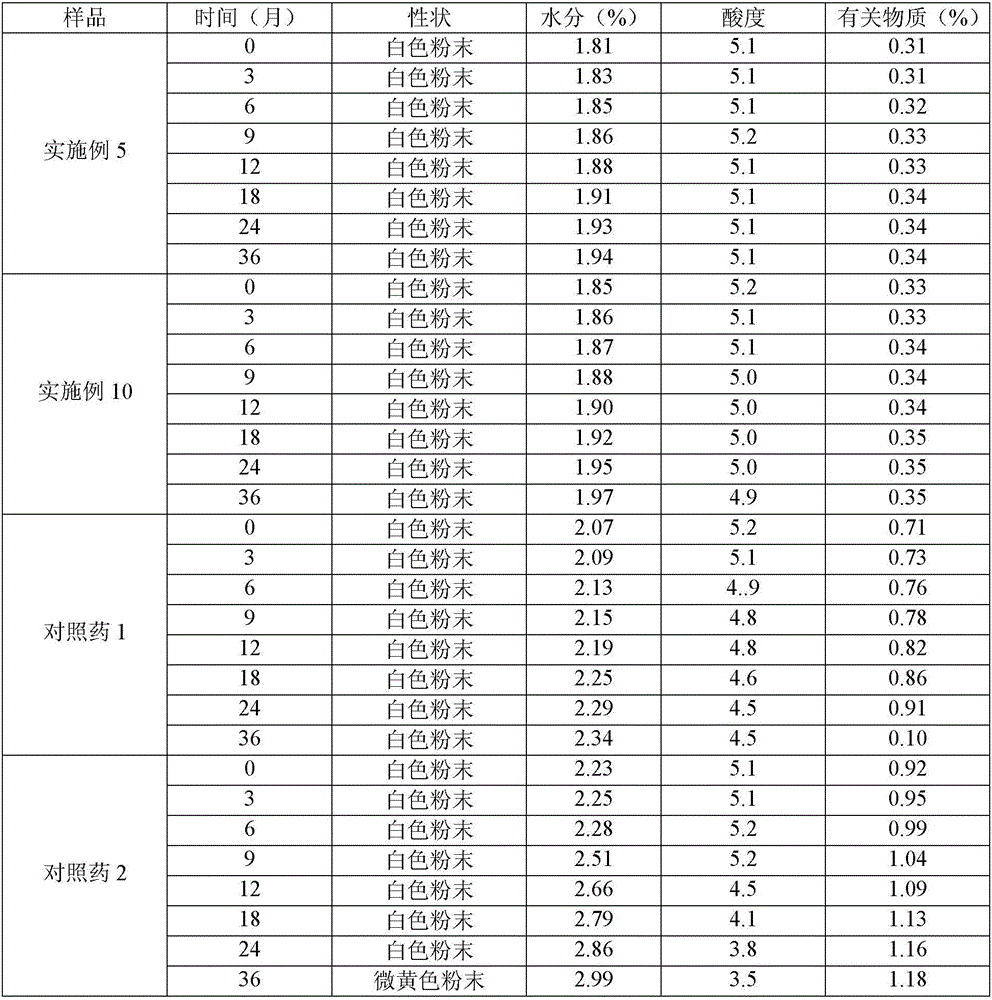
Method for evaluating stability of cefoperazone sodium leechdom
ActiveCN1971258AQuickly assess stabilityGuide productivityPreparing sample for investigationMaterial analysis using radiation diffractionStatistical analysisX-ray
The invention provides an evaluating method of stableness of cefoperazone sodium medicine; the crystal analysis is preceded by powder X-ray diffraction technology, the diffraction characteristic parameters which express the different crystal sample is selected as the quantizing index, the different crystal samples can be partied into different monoids by the statistical analysis. Based on the above, different sample monoids are associated with its stability to build the correlation relationship of the diffraction characteristic parameters and the stability, so the evaluation of stability can be rapidly realized with the diffraction characteristic parameters. The method is quick, convenient and accurate, it compensates for the disadvantages of traditional method, can do quick evaluation for the stability of the cefoperazone sodium medicine, and can be used to guide the company to produce the production and improve the process.
Owner:NAT INST FOR FOOD & DRUG CONTROL +1
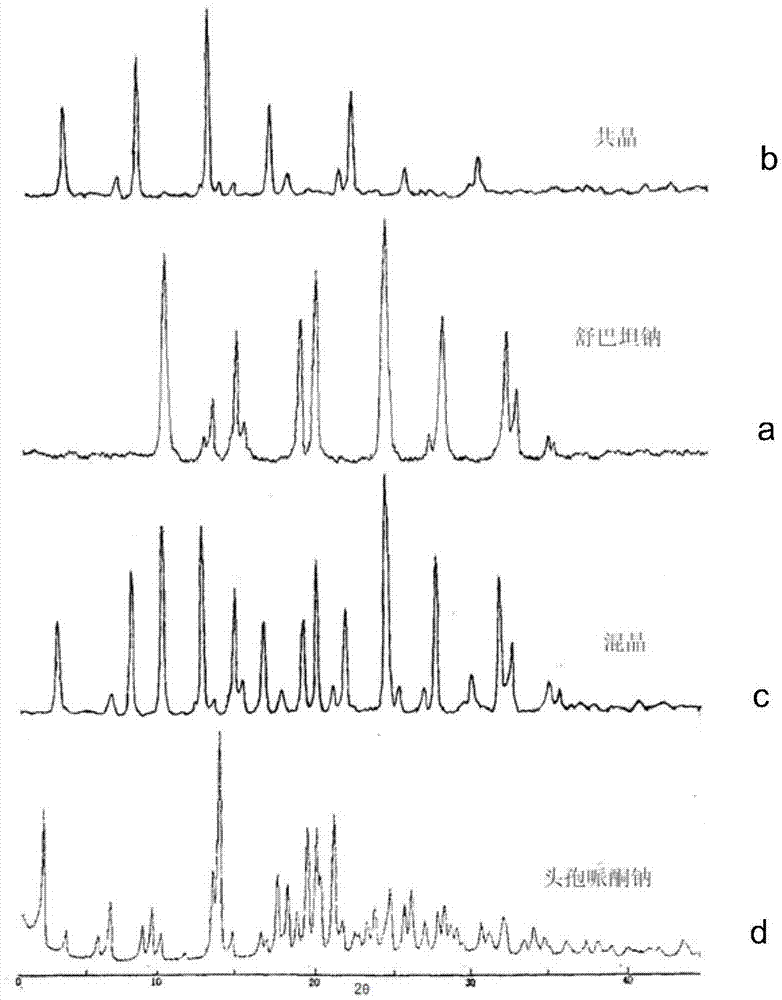
Cefoperazone sodium-sulbactam sodium eutectic crystal and composition, and preparation methods thereof
ActiveCN104844624AImprove stabilityEasy to operateAntibacterial agentsPowder deliveryPhosphateCrystallinity
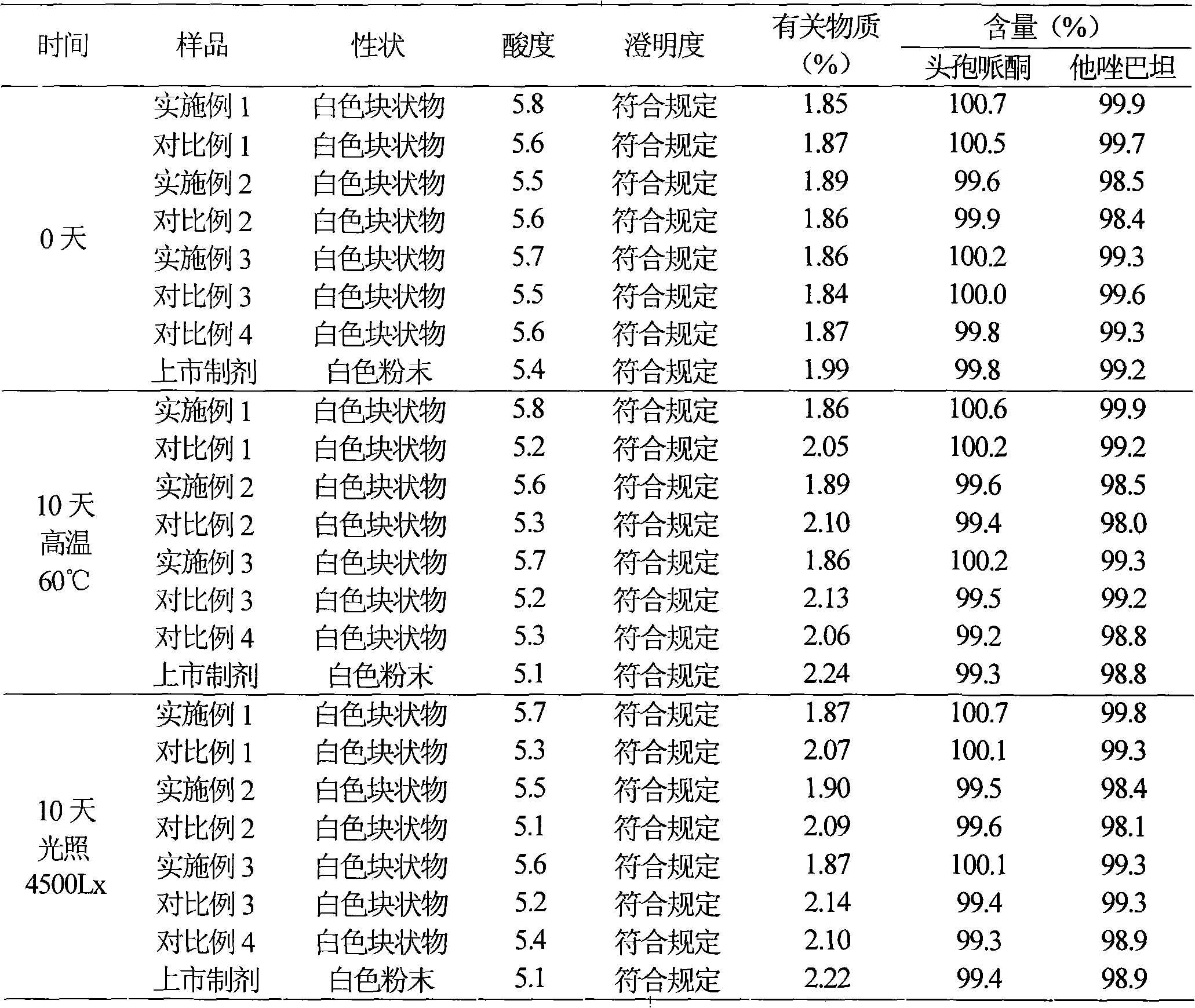
The invention relates to a cefoperazone sodium-sulbactam sodium eutectic crystal and a pharmaceutical composition containing the eutectic crystal, and preparation methods thereof. The cefoperazone sodium-sulbactam sodium eutectic crystal has the advantages of concentrated distribution of crystal form grain size, good product fluidity, gloss surface, high degree of crystallinity and good stability; the preparation method of the crystal form is simple in process, easy to operate, and suitable for popularization and application in a wide range. In the compound cefoperazone sodium-sulbactam sodium preparation, through introduction of the cefoperazone sodium-sulbactam sodium eutectic crystal and a phosphate buffer to adjust the pH value, the problems that a mixed powder in the preparation is poor in uniformity, liquidity and raw material stability are solved, generation of carbon dioxide gas due to addition of a stabilizer carbonate is avoided, an obtained powder injection is diluted through conventional transfusion, then dissolved rapidly and does not produce crystallization and degradation products, the solution clarity is in accordance with provisions, the solution pH value has no obvious change, and the contents of cefoperazone sodium and sulbactam sodium are stable.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Cefoperazone sodium and sulbactam sodium composition
InactiveCN101143146AReduce contentReduce manufacturing costAntibacterial agentsPharmaceutical delivery mechanismInfected patientCurative effect
The invention provides a combination of cefoperazone sodium and sulbactam sodium. The combination consists of the cefoperazone sodium and the sulbactam sodium, and the weight ratio of which is 3 to 1. The combination of cefoperazone sodium and sulbactam sodium of the invention has the comparative curative effect at the aspect of antibacterial effect with a compound preparation of 1 to 1 or 2 to 1 of the cefoperazone sodium / the sulbactam sodium in markets. The invention reduces the corresponding content of the sulbactam sodium in the combination, so the invention has wider clinical application range and can reduce the production cost of the drug. Compared with the prior compound preparation of the cefoperazone sodium / the sulbactam sodium, the invention is characterized by being fit for the anti-infection remedy of the patient with the renal dysfunction and the remedy of the seriously infected patient.
Owner:CHENGDU BOAOTONG TECH
Ambroxol salt
The invention relates to ambroxol salt with a chemical formula (I). Compared with ambroxol hydrochloride, the ambroxol salt has better solubility and faster dissolving speed and can be compatible with cefoperazone sodium for using.
Owner:沈阳华泰药物研究有限公司
Drug composition of cefoperazone sodium and tazobactam sodium and preparation method thereof
InactiveCN103230400AReduce humidityImprove thermal stabilityAntibacterial agentsOrganic chemistryThermal stabilityStructural formula
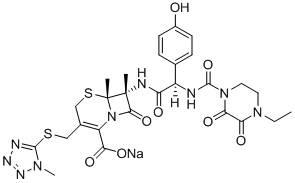
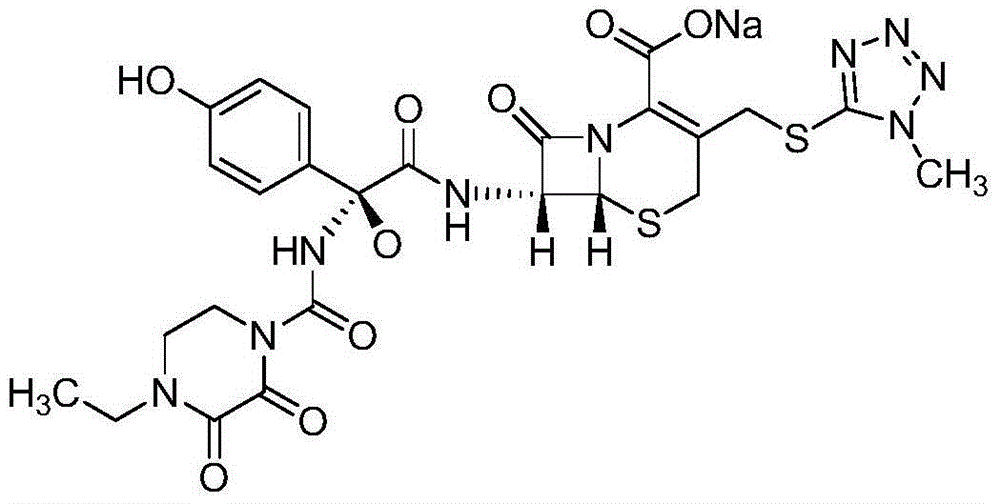
The invention belongs to the technical field of medicines and in particular relates to a drug composition of cefoperazone sodium and tazobactam sodium and a preparation method thereof. The drug composition is sterile powder injection. The mass ratio of cefoperazone sodium to tazobactam sodium in the drug composition is (4-8):1, wherein cefoperazone sodium is shown in a drawing 1, an X-ray powder diffraction pattern obtained by powder X-ray diffractometry and the chemical structural formula of cefoperazone sodium is shown in the formula (I) in the specification. Compared with the prior art, the cefoperazone sodium compound used in the drug composition has lower hygroscopicity and good thermal stability, thus improving the stability of the drug composition of cefoperazone sodium and tazobactam sodium and reducing the impurity content.
Owner:四川省惠达药业有限公司
Ambroxol derivative and application
ActiveCN105693764AImprove solubilityWide pH solubility rangeOrganic compound preparationGroup 5/15 element organic compoundsSolubilityBioavailability
The invention relates to the field of medicinal chemistry, in particular to an ambroxol derivative and a preparation method thereof and application of the ambroxol derivative in drugs for eliminating phlegm. The ambroxol derivative is a compound shown in a structure in the description or a stereoisomer, and please refer to figure 1 for the specific structure, wherein R1 represents H or alkali metal ions or organic amine, and R2 represents H or alkali metal ions or organic amine. The ambroxol derivative has good solubility, the solution pH is larger than 7, no sediment is separated out, the ambroxol derivative can be used concomitantly with cefoperazone sodium, a wider pH solubility range is achieved, the oral bioavailability is high, and the drug efficacy is greatly strengthened.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Powder mixing technology for compound antibiotic
ActiveCN103040768AReduce pollutionStable chemical qualityPowder deliveryAntiinfectivesChemical qualityOrganic solvent
The invention relates to a powder mixing technology for a compound antibiotic. The compound antibiotic is obtained by cold drying after uniform mixing of antibiotic A and antibiotic B in advance, wherein the antibiotic A is mezlocillin sodium, amoxicillin sodium, cefoperazone sodium, and piperacillin sodium, and the antibiotic B is sulbactam sodium. According to the powder mixing technology, organic solvent is omitted, the environment is less polluted, the freeze-drying technology has stable yield, and the cost is low; the freeze-drying mixed powder has good uniformity, fast dissolution rate, and stable chemical quality, so that the powder mixing technology is applicable to preparation of various of compound antibiotics, and is suitable for mass production.
Owner:山东二叶制药有限公司
Stable cefoperazone medicine prparation
InactiveCN101036657AImprove product qualitySignificant changeAntibacterial agentsOrganic active ingredientsSodium bicarbonateSolvent
The invention discloses a stable compound preparation of cefoperazone drug, which is comprised by cefoperazone acid and latent solvent, which weight ratio is 1:0.62~0.06. The latent solvent is preferred selected from sodium carbonate and sodium bicarbonate. Related substances of the compound preparation in the invention and labelled content do not change much in influencing factor and accelerated test in 40 DEG C., which accord with the standard of pharmacopoeia, and the product quality is more stable than cefoperazone sodium during period of validity.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Cefoperazone sodium and sulbactam sodium composition
ActiveCN105853441AHigh lattice energyImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsSulbactam SodiumCefbuperazone
The invention provides a cefoperazone sodium and sulbactam sodium composition, which comprises the following raw materials in parts by weight: 9-10 parts of cefoperazone sodium, 1 part of sulbactam sodium and 0.05-0.07 part of glutathione. The glutathione is added to the composition, so that the composition has relatively good antibacterial effect. Furthermore, a little of sulbactam sodium is utilized, so that accumulation of the sulbactam sodium in a body is reduced; and the production cost is reduced. In addition, by a novel sulbactam sodium crystal, the composition provided by the invention has relatively good stability.
Owner:HAINAN HERUI PHARMA
Cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection
ActiveCN105748482AImprove solubilityLight colorAntibacterial agentsPowder deliverySodium bicarbonateSolubility
The invention belongs to the technical field of medicines, and particularly relates to cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection and a preparation method of the cefoperazone sodium and tazobactam sodium pharmaceutical composition. The pharmaceutical composition is a freeze-dried powder preparation. The mass ratio of cefoperazone sodium to tazobactam sodium in the pharmaceutical composition is 4:1. The preparation method comprises the steps as follows: firstly, tazobactam is refined; then, tazobactam sodium is prepared from refined tazobactam and sodium bicarbonate; finally, cefoperazone sodium and prepared tazobactam sodium are uniformly mixed for preparation of freeze-dried powder. Compared with like products sold in the market, the pharmaceutical composition has the advantages of good solubility, light color, few related substances, small polymer content and low adverse reaction rate; besides, effective constituents in the pharmaceutical composition are stable, and when the pharmaceutical composition is preserved for a long term, few effective constituents are degraded, the content of impurities is low, the quality performance of products is relatively good, and the medication safety of patients is guaranteed accordingly.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com