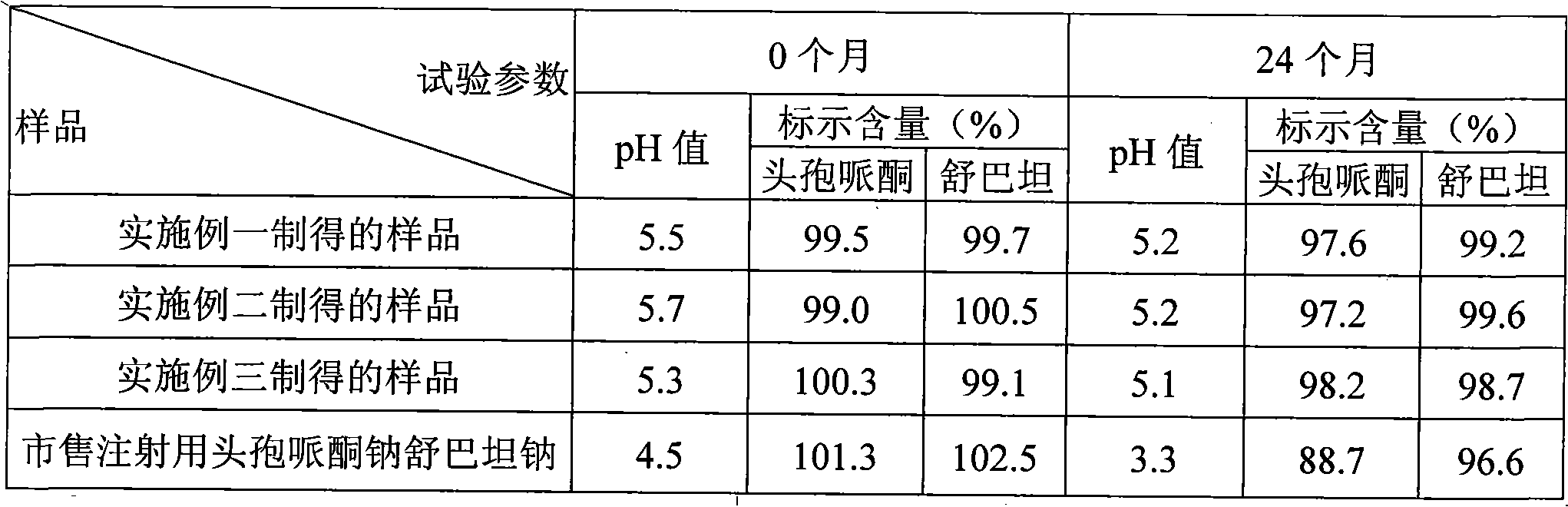
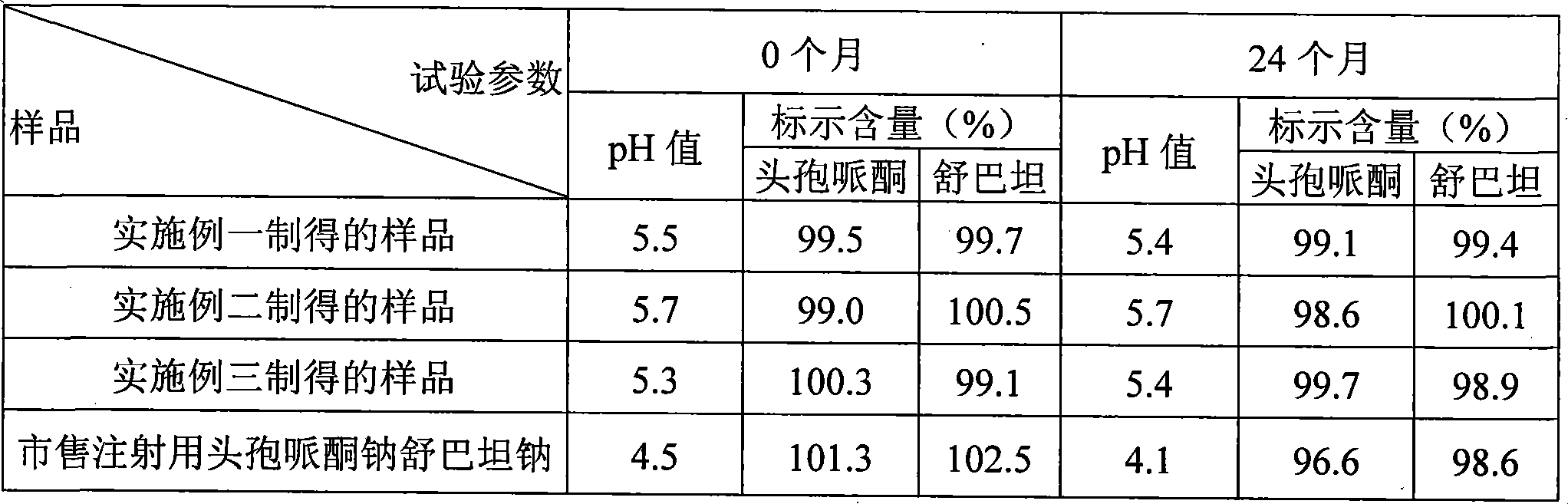
Cefoperazone sodium and sulbactam sodium combination and preparation method thereof
A technology of cefoperazone sodium sulbactam sodium and cefoperazone sodium sulbactam sodium, which is applied in the field of compound preparations of cefoperazone sodium, can solve the problems of poor stability of cefoperazone sodium and no great improvement in stability, and achieve simple steps and improved Stable, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Prescription A
[0024] Cefoperazone Sodium 100.0g
[0025] Sulbactam Sodium 100.0g
[0026] Sodium dihydrogen phosphate 3.5g
[0027] Disodium hydrogen phosphate 5.4g
[0028] Sodium chloride 3.4g
[0029] Purified water to 500ml
[0030] A total of 100 bottles were produced
[0031] 2. Preparation method
[0032] Step 1: take cefoperazone sodium, sulbactam sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride by prescription quantity;
[0033] Step 2: add water to dissolve, and adjust the pH value to 5.0-6.5 with phosphoric acid or sodium hydroxide solution;
[0034] Step 3: The prepared solution is adsorbed by activated carbon and filtered through a microporous membrane, then filled, and finally freeze-dried to obtain a cefoperazone sodium-sulbactam sodium composition, and made into an injection.
Embodiment 2
[0036] 1. Prescription B
[0037] Cefoperazone Sodium 100.0g
[0038] Sulbactam Sodium 50.0g
[0039] Sodium dihydrogen phosphate 3.5g
[0040] Disodium hydrogen phosphate 5.4g
[0041] Sodium chloride 3.4g
[0042] Purified water to 400ml
[0043] A total of 100 bottles were produced
[0044] 2. Preparation method
[0045] Step 1: take cefoperazone sodium, sulbactam sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride by prescription quantity;
[0046] Step 2: add water to dissolve, and adjust the pH value to 5.0-6.5 with phosphoric acid or sodium hydroxide solution;
[0047] Step 3: The prepared solution is adsorbed by activated carbon and filtered by a microporous membrane, then loaded into a large plate, freeze-dried, pulverized, sieved, and then aseptically packaged to prepare a compound preparation of cefoperazone sodium and sulbactam sodium for injection.
Embodiment 3
[0049] 1. Prescription C
[0050] Cefoperazone Sodium 100.0g
[0051] Sulbactam Sodium 25.0g
[0052] Sodium dihydrogen phosphate 3.5g
[0053] Disodium hydrogen phosphate 5.4g
[0054] Sodium chloride 3.4g
[0055] Purified water to 400ml
[0056] A total of 100 bottles were produced
[0057] 2. Preparation method
[0058] The first step: take cefoperazone sodium, sulbactam sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride by prescription quantity;
[0059] The second step: add water to dissolve, adjust the pH value to 5.0-6.5 with phosphoric acid or sodium hydroxide solution, then fill it after activated carbon adsorption and microporous membrane filtration, and finally freeze-dry to make cefoperazone sodium sulbactam for injection Sodium compound preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com