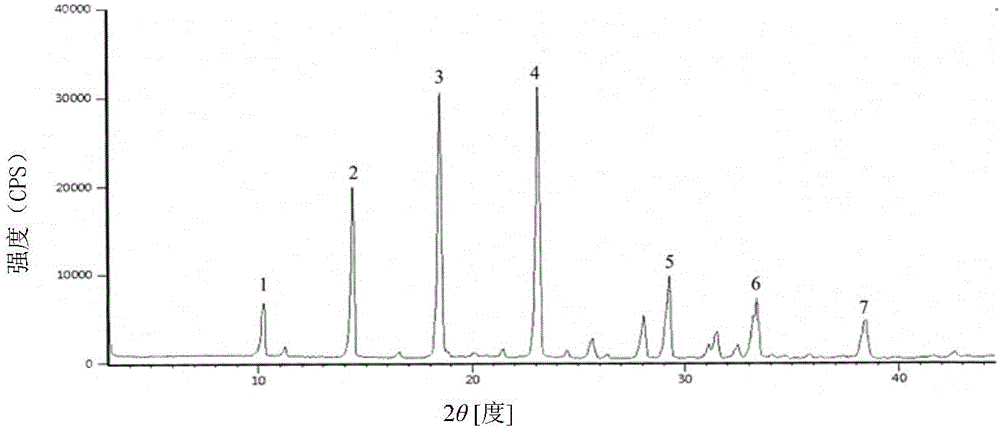
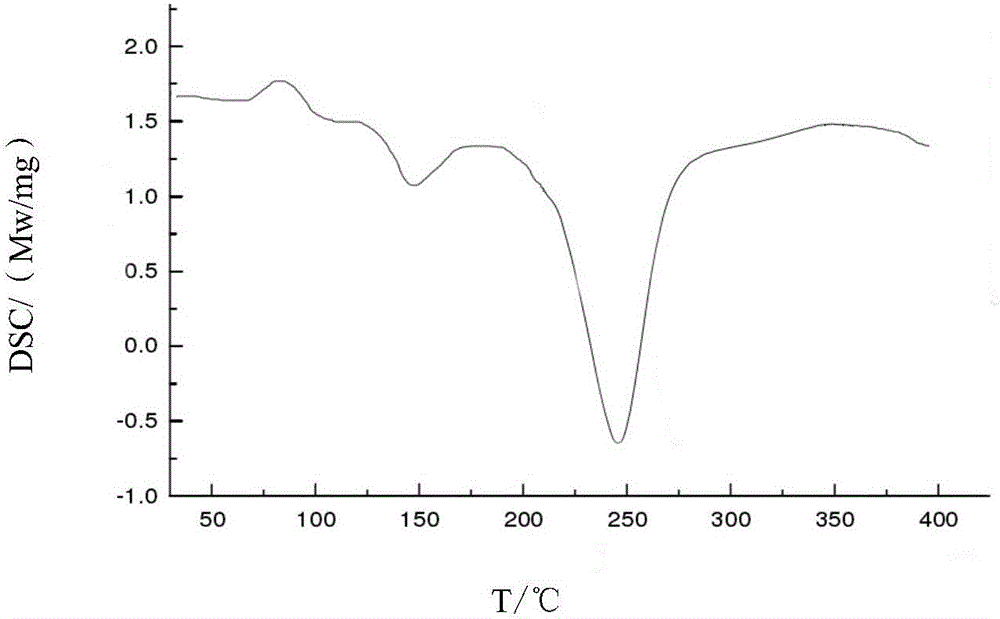
Cefoperazone sodium compound prepared by using fluid mechanics principle and preparation comprising cefoperazone sodium compound
A kind of technology of cefoperazone sodium and compound, which is applied in the field of cefoperazone sodium compound and its preparation, and can solve problems such as potential safety hazard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of cefoperazone sodium compound
[0036] making process:
[0037]Measure 500ml of ethyl acetate and 100ml of water and add them to the standing wave reactor of the hydrodynamic principle device. While stirring, 25.01 g of sodium carbonate was slowly added. Adjust the pH value to 6.2. After the pH value is stable, add 0.21 g of activated carbon, decolorize for 30 min, and filter. Wash the carbon cake successively with acetone 50ml×2 and water 50ml×2. The temperature of the solvent circulation tank is controlled at 18-20°C, the temperature of the standing wave reactor is controlled at 30-35°C, the circulation pump is turned on, and 300ml of acetone is added dropwise to the standing wave reactor. Crystallize for 30min. Slowly lower the temperature of the solvent circulation tank to 8-10°C, turn on the circulation pump, continue to add 50ml of acetone dropwise, turn off the circulation pump, and stand for crystallization for 2 hours. Sucti...
Embodiment 2
[0039] Embodiment 2: the preparation of cefoperazone sodium compound
[0040] Measure 600ml of ethyl acetate and 100ml of water and add them to the standing wave reactor of the hydrodynamic principle device. While stirring, 25.02 g of sodium carbonate was slowly added. Adjust the pH value to 5.1, and after the pH value is stable, add 0.20 g of activated carbon, decolorize for 30 min, and filter. Wash the carbon cake successively with acetone 50ml×2 and water 50ml×2. The temperature of the solvent circulation tank is controlled at 15-18°C, the temperature of the standing wave reactor is controlled at 30-35°C, the circulation pump is turned on, and 300ml of acetone is added dropwise to the standing wave reactor. Crystallize for 30min. Slowly lower the temperature of the solvent circulation tank to 5-8°C, turn on the circulation pump, continue to add 50ml of acetone dropwise, turn off the circulation pump, and let it stand for crystallization for 2 hours. Suction filtration, ...
Embodiment 3
[0042] Embodiment 3: the preparation of cefoperazone sodium compound
[0043] Measure 400ml of ethyl acetate and 100ml of water and add them to the standing wave reactor of the hydrodynamic principle device. While stirring, 25.04 g of sodium carbonate was slowly added. Adjust the pH value to 6.5. After the pH value is stable, add 0.25 g of activated carbon, decolorize for 30 min, and filter. Wash the carbon cake successively with acetone 50ml×2 and water 50ml×2. The temperature of the solvent circulation tank is controlled at 10-15°C, the temperature of the standing wave reactor is controlled at 30-35°C, the circulation pump is turned on, and 300ml of acetone is added dropwise to the standing wave reactor. Crystallize for 30min. Slowly lower the temperature of the solvent circulation tank to 2-5°C, turn on the circulation pump, continue to add 50ml of acetone dropwise, turn off the circulation pump, and let it stand for crystallization for 2h. Suction filtration, and the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com