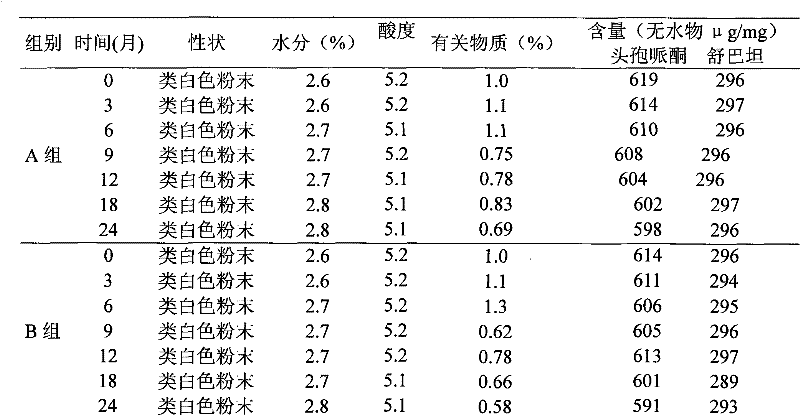
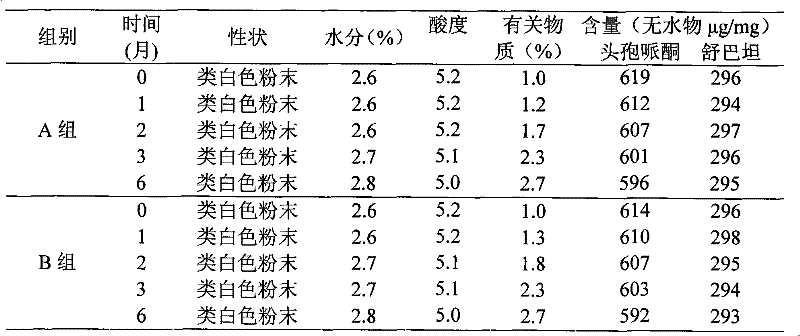
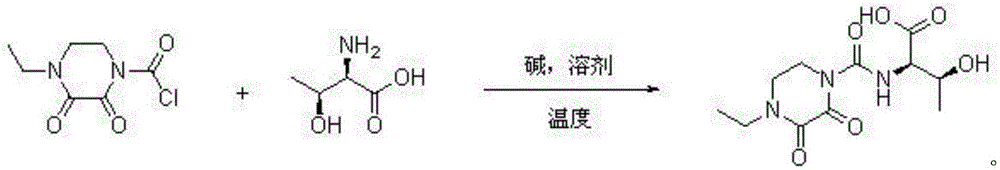
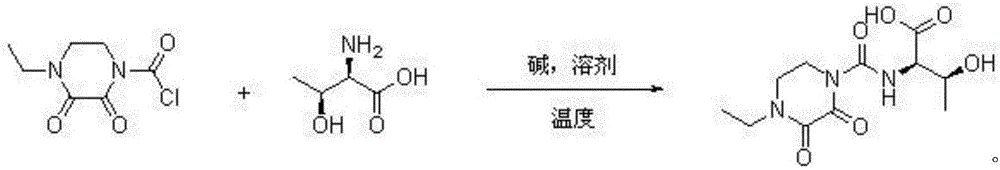
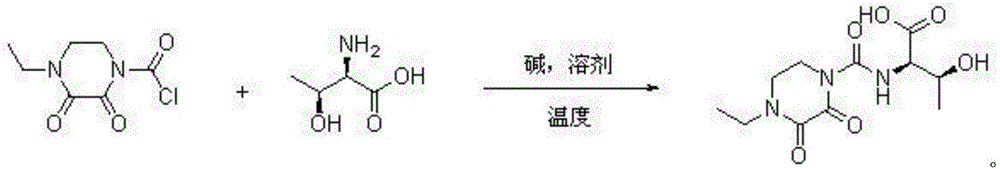
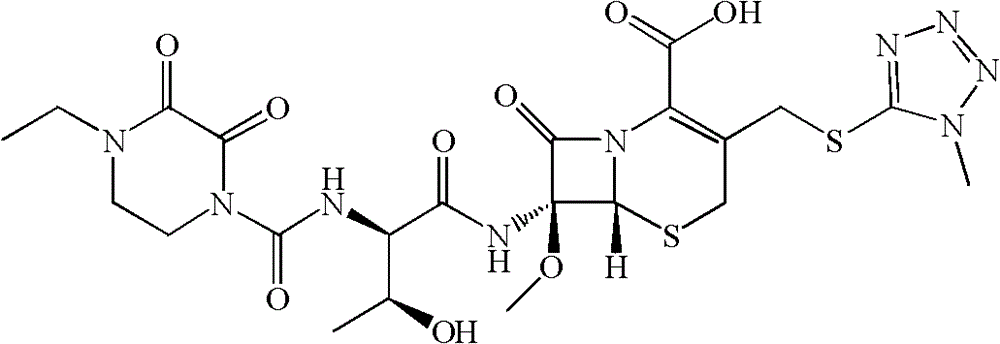
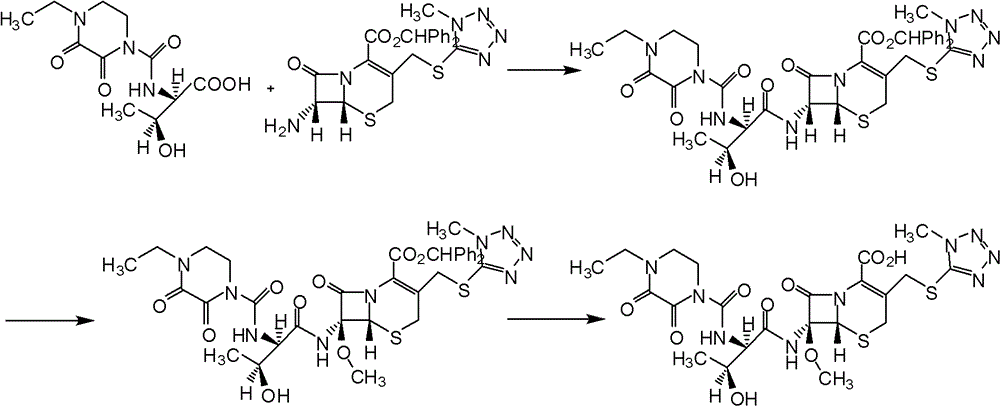
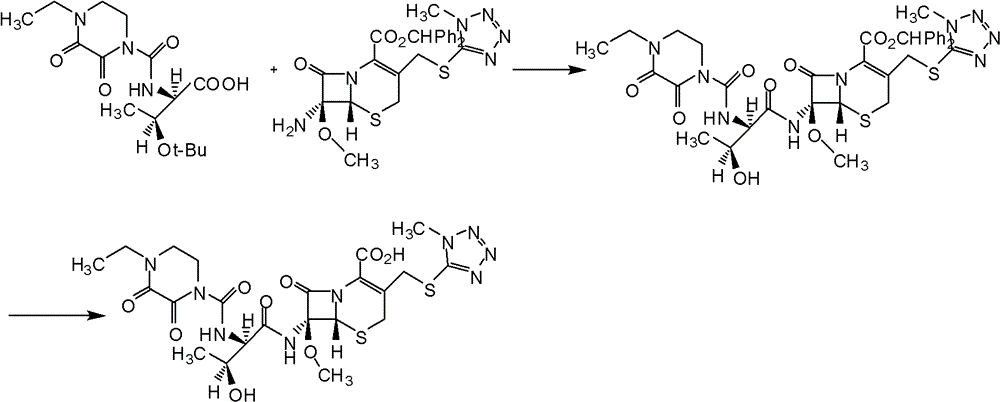
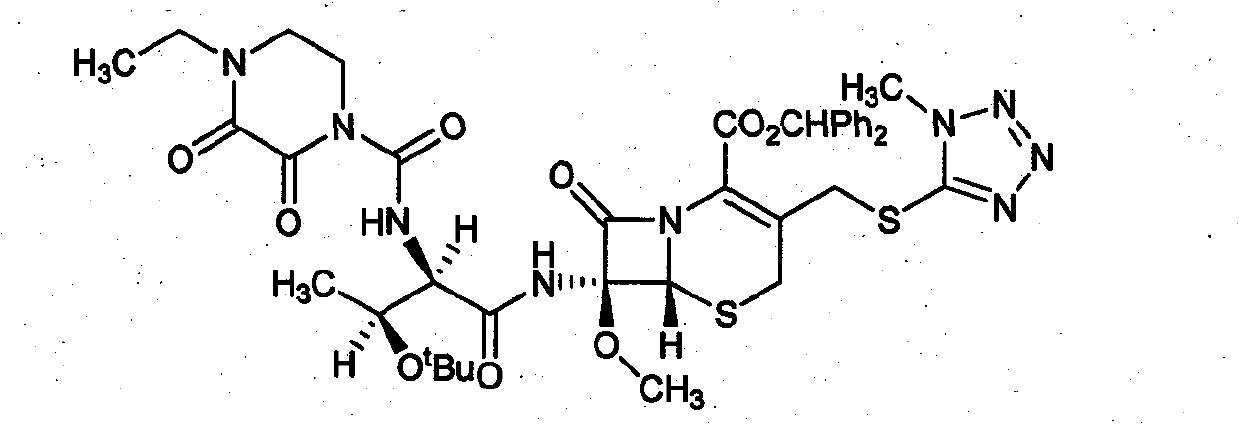
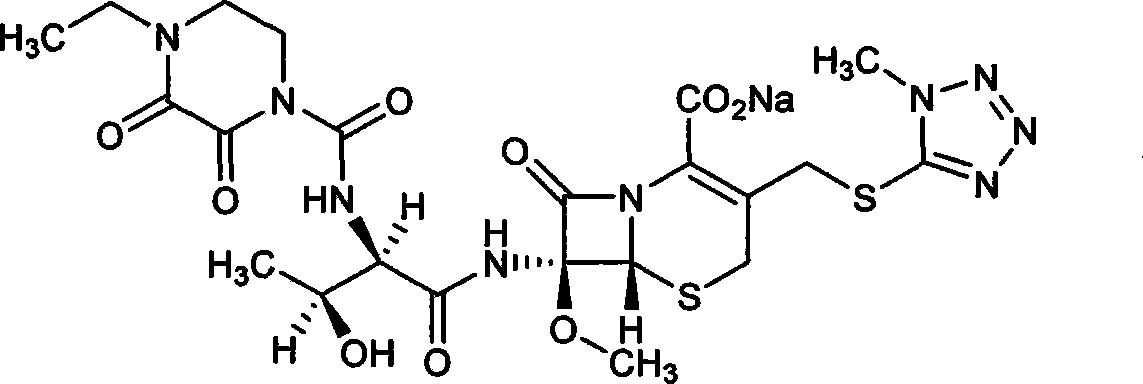
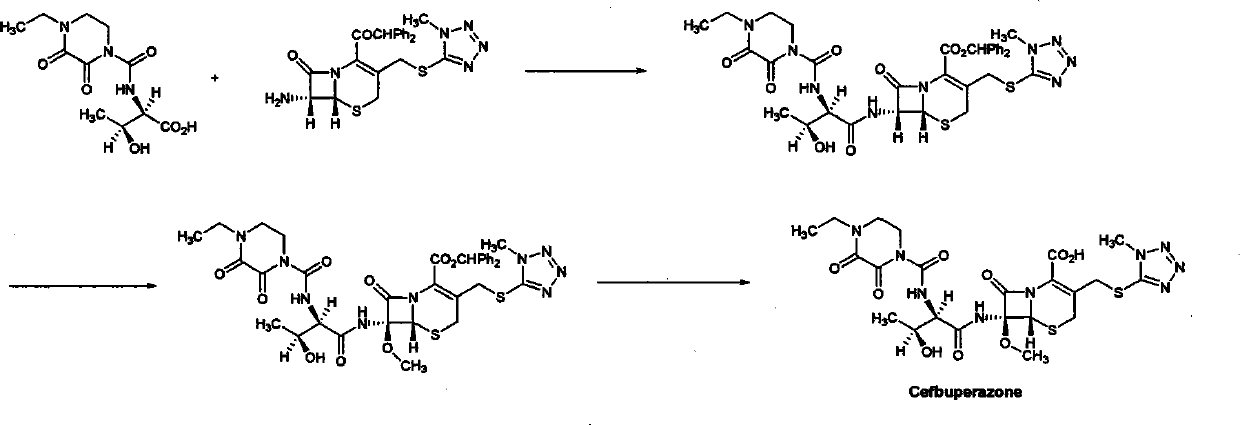
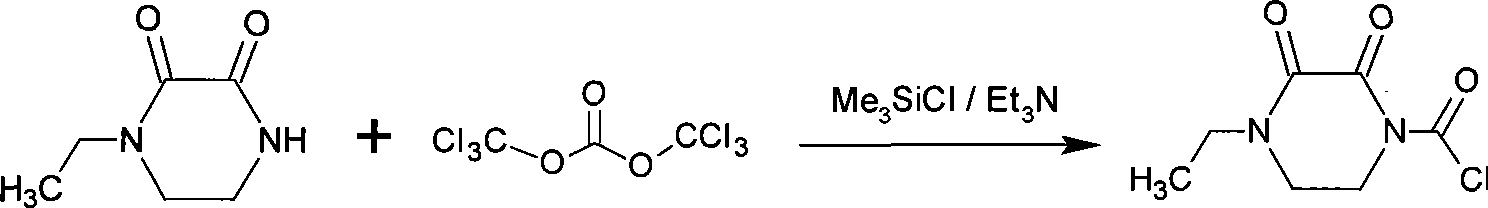Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Cefbuperazone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
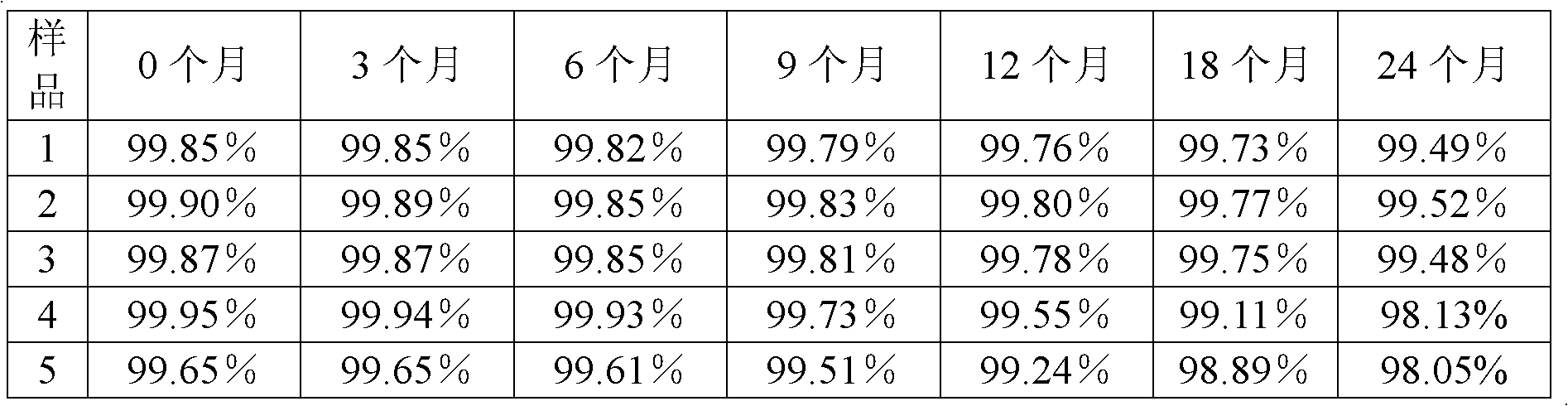
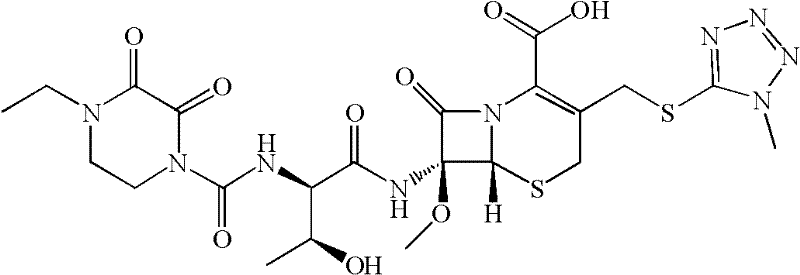
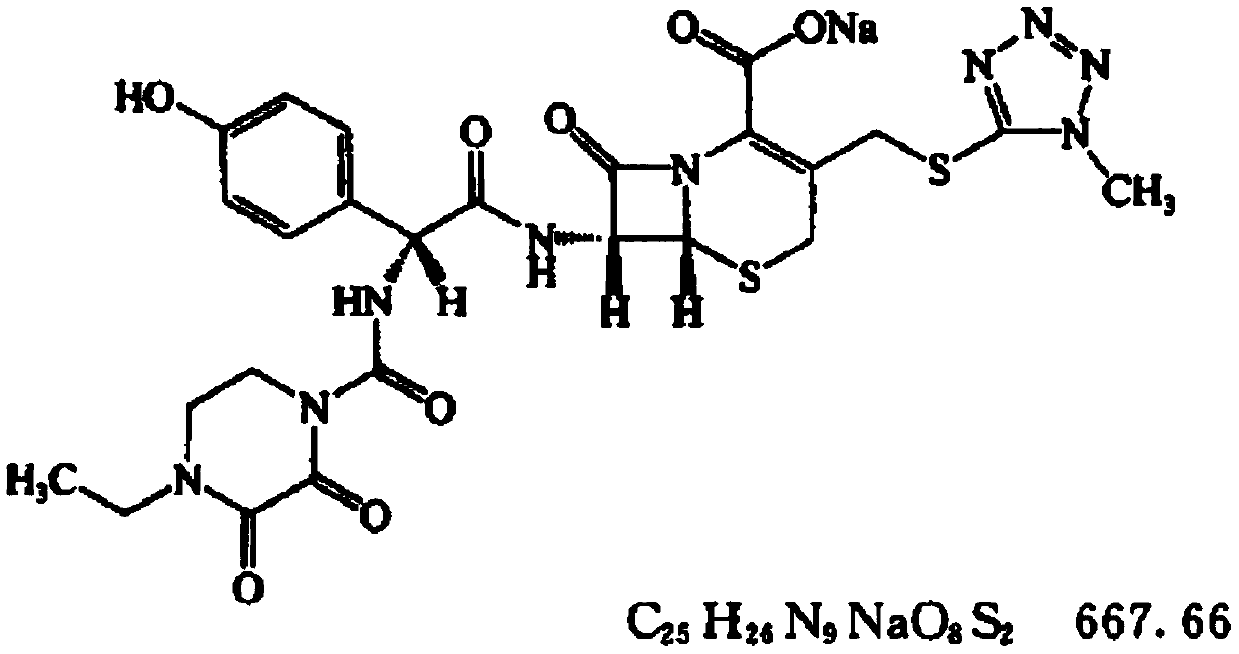
Cefbuperazone (INN) is a second-generation cephalosporin antibiotic.
A kind of cefoperazone sodium tazobactam sodium pharmaceutical composition
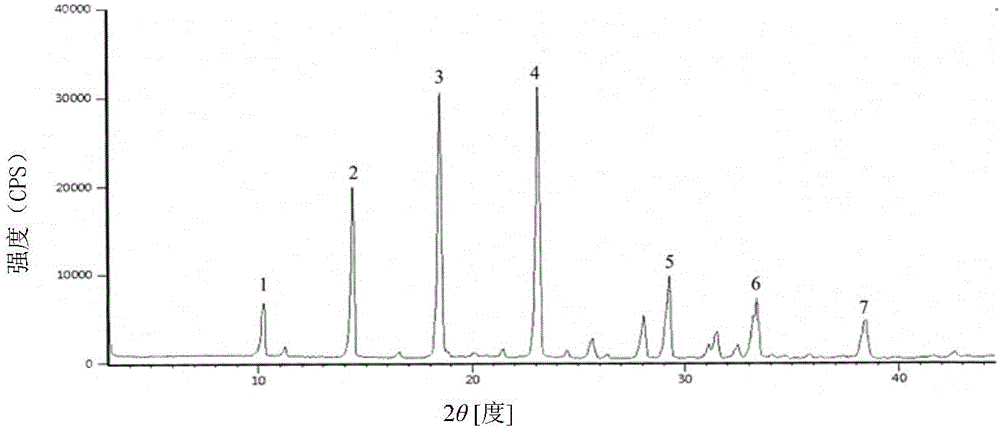
ActiveCN102274233AReduce security risksFew kindsAntibacterial agentsHeterocyclic compound active ingredientsPowder diffractionSubstance content
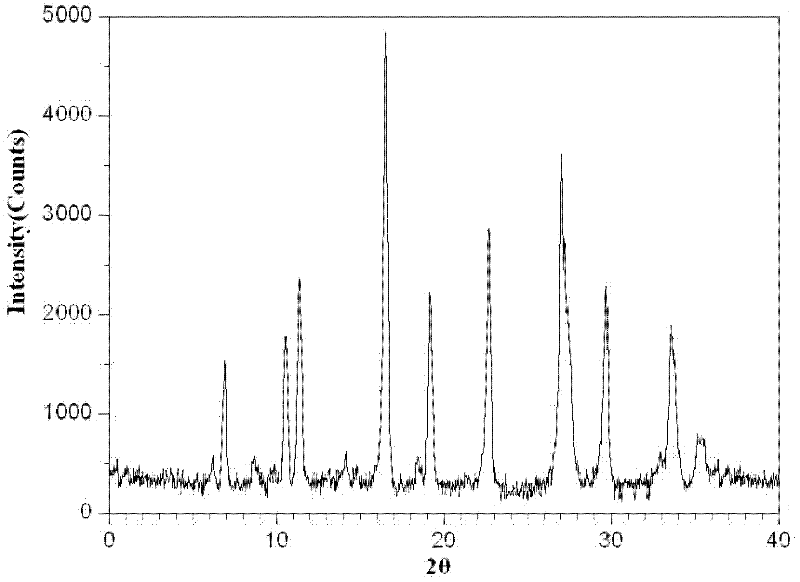
The invention relates to a medicinal composition of cefoperazone sodium and tazobactam sodium. The medicinal composition comprises the following components in part by weight: 4 to 8 parts of cefoperazone sodium and 1 part of tazobactam sodium, wherein the tazobactam sodium is measured by a powder X-ray diffraction measuring method, and characteristic diffraction peaks are shown at the positions of 6.9 degrees, 10.5 degrees, 11.4 degrees, 16.6 degrees, 19.2 degrees, 22.7 degrees, 27.0 degrees, 29.7 degrees and 33.5 degrees in an X-ray powder diffraction map expressed by a diffraction angle of between 2 theta+ / -0.2 degree. The medicinal composition has the advantages of high stability, low relevant substance content, controllable quality and the like, and the administration safety of patients is improved. The invention also relates to a method for preparing the tazobactam sodium with the technical characteristics of the characteristic diffraction peaks.
Owner:江西璟瑞药业有限公司
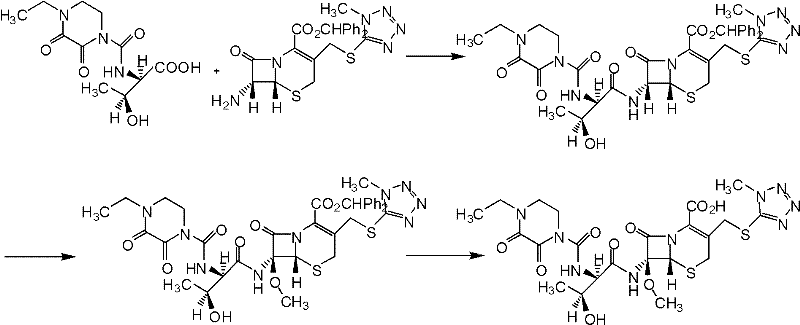
Synthesis method of cefbuperazone
InactiveCN102250124ASimple designReduce pollutionOrganic chemistryBulk chemical productionTrimethylsilyl chlorideSilanes
The invention relates to a synthesis method of cefbuperazone, and the synthesis method comprises the following steps: (1) protecting 7beta-amino-7alpha-methoxy-3-[(1-methyl-1H-tetrazol-5-yl)S-methyl]-3-cephalosporin-en-4-carboxylic acid diphenyl methyl ester (7-MAC) with an amino group protective agent to obtain 7-MAC silane; (2) reacting cefbuperazone lateral chain with a halide to obtain cefbuperazone lateral chain acyl halide; (3) reacting 7-MAC silane with cefbuperazone lateral chain acyl halide to obtain an intermediate product, namely cefbuperazone diphenyl methyl ester; and (4) removing the protective group from cefbuperazone diphenyl methyl ester to obtain cefbuperazone, wherein the amino group protective group is hexamethyl disilylamine, trimethyl chlorosilane, N,O-bis(trimethylsilyl) acetamide or N,N-bis(trimethylsilyl)-2,2,2-trifluoroacetamide. The synthesis method is easy for realization and has the advantages of simple and reasonable synthesis route design, mild reaction conditions, simple, safe and controllable reaction process and high yield of cefbuperazone, and the intermediate product is easy to separate and purify.
Owner:HAINAN HERUI PHARMA +1
Composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium and preparation process thereof
ActiveCN104013629AImprove solubilityImprove stabilityAntibacterial agentsRespiratory disorderSolubilityAntioxidant
The invention discloses a composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium. The composite pharmaceutical composition is an injection, and is prepared from the following components in parts by weight: 3.5-4.5 parts of cefoperazone sodium, 1 part of tazobactam sodium, 0.5-1 part of ambroxol hydrochloride, 0-6 parts of excipient, 0-10 parts of isotonic agent, 0-1 part of antioxidant, a proper amount of citric acid-sodium citrate and 20 parts of injection water. The composite preparation of the cefoperazone sodium and the tazobactam sodium disclosed by the invention is stable in quality and significant in curative effect, not only can three active ingredients be evenly mixed, but also the composite pharmaceutical composition is excellent in stability, good in solubleness and good in clinical use safety.
Owner:福安药业集团庆余堂制药有限公司
Cefoperazone sodium compound prepared by using fluid mechanics principle and preparation comprising cefoperazone sodium compound
InactiveCN106432273AEasy to manufactureSmall particle sizeOrganic active ingredientsOrganic chemistryMedical productX-ray
The invention discloses a cefoperazone sodium compound prepared by using the fluid mechanics principle. Research&Development and Industrialization Project of High-end Medical Product Refinement Crystallization Technologies wins the second prize of national scientific and technological progress in 2015, and the fluid mechanics principle crystallization technology belongs to one of the high-end medical product refinement crystallization technologies. The cefoperazone sodium compound is determined by using X-ray powder diffraction, and the main characteristic peaks represented by the diffraction angle 2 theta in a map are located at 10.25+ / -0.2 degrees, 14.40+ / -0.2 degrees, 18.51+ / -0.2 degrees, 23.14+ / -0.2 degrees, 29.10+ / -0.2 degrees, 33.25+ / -0.2 degrees and 38.45+ / -0.2 degrees. Cefoperazone acid reacts with a salt forming agent, and the cefoperazone sodium compound is prepared through secondary crystallization. The operation is simple, reactants are easy to obtain, the reaction condition is mild, and the yield is high. The compound is high in purity, low in impurity content, good in fluidity and good in stability. Meanwhile, the invention further discloses a preparation prepared from cefoperazone sodium, namely, cefoperazone sodium for injection. The preparation process of the preparation is simple, no excipient is needed, and the preparation has better stability and few side effects.
Owner:陕西顿斯制药有限公司
Preparation method for injection cefoperazone sodium tazobactam sodium composition
InactiveCN103120692AGuaranteed uniformityAntibacterial agentsPowder deliveryCefbuperazoneTAZOBACTAM SODIUM
The invention provides a preparation method for injection cefoperazone sodium tazobactam sodium composition. The preparation method comprises the following steps of: crushing the cefoperazone sodium and the tazobactam sodium; and mixing the cefoperazone sodium and the tazobactam sodium in a weight ratio of 4:1; and separately packaging, pressing and capping the mixture. The preparation method is characterized in that the fineness of pulverization of the cefoperazone sodium and the tazobactam sodium is 50-120 meshes.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Preparation method of cefoperazone intermediate 7-TMCA
InactiveCN102627659AImprove performanceSafe and reliable performanceOrganic chemistryBoron trifluorideAcetonitrile
The invention provides a preparation method of cefoperazone intermediate 7-TMCA. The preparation method comprises the following steps of: adding a boron trifluoride dimethyl carbonate complex and dimethyl carbonate into a first 1L reaction bottle A; starting to stir; throwing methyl mercaptotetrazole into the bottle when the temperature is reduced to 10-15 DEG C; stirring for 10-30 minutes to fully dissolve the methyl mercaptotetrazole; transferring reaction solution from the first 1L reaction bottle A into a second 1L reaction bottle B in which de-ionized water is held; and drying under a vacuum condition at 80 DEG C for 3-4 hours and taking out. According to the preparation method, the dimethyl carbonate is used as a solvent in place of acetonitrile, the boron trifluoride dimethyl carbonate complex is used as a catalyst in place of a boron trifluoride acetonitrile complex, and the dimethyl carbonate is non-toxic, has similar physical property to that of water, is safe and convenient to use, has less pollution and is easy to transport. An obtained product has a better crystal form and is not required to be crushed in a drying step; and the drying time of the product can be shortened.
Owner:HEILONGJIANG HAOYUN FINE CHEM
Method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate
The invention discloses a method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate. In the method, 1-ethyl-2,3-dioxypiperazine and chloro-formate are used as raw materials and reacted in an organic solvent system in the presence of an acid binding agent to form 4-ethyl-2,3-dioxypiperazine-1-formate, wherein the molar ratio of the 1-ethyl-2,3-dioxypiperazine to the acid binding agent to the chloro-formate is 1:1.0-3.0:1.0-2.0; the chloro-formate is methyl chloroformate or ethyl chloroformate; and the 4-ethyl-2,3-dioxypiperazine-1-formate is 4-ethyl-2,3-dioxypiperazine-1-methyl formate or 4-ethyl-2,3-dioxypiperazine-1-ethyl formate. The method greatly reduces cost, simplifies process, reduces byproducts, improves product purity and reduces solvent separation processes; and the prepared product can be used as an intermediate for piperacillin and cefoperazone and is suitable for industrial production.
Owner:山东艾孚特科技有限公司
Cefoperazone sodium and sulbactam sodium composition
ActiveCN105853441AHigh lattice energyImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsSulbactam SodiumCefbuperazone
The invention provides a cefoperazone sodium and sulbactam sodium composition, which comprises the following raw materials in parts by weight: 9-10 parts of cefoperazone sodium, 1 part of sulbactam sodium and 0.05-0.07 part of glutathione. The glutathione is added to the composition, so that the composition has relatively good antibacterial effect. Furthermore, a little of sulbactam sodium is utilized, so that accumulation of the sulbactam sodium in a body is reduced; and the production cost is reduced. In addition, by a novel sulbactam sodium crystal, the composition provided by the invention has relatively good stability.
Owner:HAINAN HERUI PHARMA
Preparation method of high-purity cefoperazone sodium micro powder
ActiveCN109796470AHigh purityLess impuritiesOrganic chemistryBulk chemical productionImpurityCrystallization
The invention discloses a preparation method of high-purity cefoperazone sodium micro powder, and belongs to the technical field of medicines. Cefoperazone acid is taken as a raw material, a cefoperazone sodium solution is prepared first, decolorizing treatment is then conducted, the decolorized cefoperazone sodium solution is added to a reaction kettle to which a super-critical fluid is added, and the cefoperazone sodium micro powder is obtained through crystallization. The preparation method has the advantages that the process is stable, the micro powder is green and environmentally friendly, the oxidation of a product can be avoided, the prepared cefoperazone sodium micro powder has the advantages of high purity and less impurity, the particle fluidity of the product is good, and the granularity is controllable.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Synthesis of 4-ethyl-(2,3-dioxo piperazinyl)formyl chloride-1 and preparation of crystal thereof
The invention relates to a method for synthesizing 4-ethyl-(2, 3-dioxo)-1-piperazine formyl chloride, which comprises the following steps: using 4-ethyl-2, 3-dioxopiperazine as a starting raw material, di-(trichloromethyl) carbonate as a formyl chloridizing reagent, trimethyl chlorosilane as an activator and a prptectant, triethylamine as an acid-binding agent and a catalyst which are reacted with each other to prepare the 4-ethyl-(2, 3-dioxo)-1-piperazine formyl chloride; and adding crystallizing agents such as n-hexane, petroleum ether, hexamethyl disiloxane, dipropyl ether, cyclohexane into a reaction liquid of the 4-ethyl-(2, 3-dioxo)-1-piperazine formyl chloride to separate out 4-ethyl-(2, 3-dioxo)-1-piperazine formyl chloride crystal. The method for synthesizing and crystallizing the 4-ethyl-(2, 3-dioxo)-1-piperazine formyl chloride provides a more appropriate intermediate for producing piperacillin and cefoperazone, and can shorten the synthesizing process and improve the quality thereof.
Owner:山西新天源药业有限公司
Preparation method of cefoperazone acid
The invention discloses a preparation method of cefoperazone acid, and belongs to the field of preparation of medicinal intermediates. The preparation method comprises the following steps: a precipitating agent is added to a solution of cefoperazone sodium used as a raw material in order to decolorize the solution, a dispersant is added to the decolorized solution, and crystallization is carried out to obtain the cefoperazone acid. The method allows the cefoperazone sodium which does not meet quality standards to be fully recycled, the prepared cefoperazone acid has the advantages of high content, few impurities and good stability, and the preparation method has the advantages of simplicity, energy saving and environmental protection, and is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
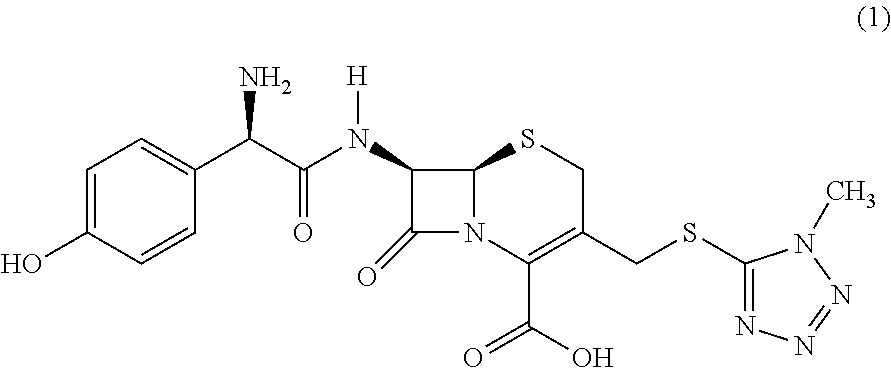
Novel crystalline cefoperazone intermediate
The present invention relates to a crystalline form of an intermediate for cefoperazone of formula (1) and to a process for the preparation thereof by enzymatic condensation of a 3′-thiosubstituted β-lactam nucleus with a phenylglycine derivative.
Owner:DSM SINOCHEM PHARMA NETHERLANDS
Preparation method for cefbuperazone
The invention provides a preparation method for cefbuperazone. The preparation method comprises the steps of performing an acylchloriration reaction on D-alpha-(4-ethyl-2 ,3-dioxo-piperazine-carboxamido)-beta-(S) hydroxybutyric acid and oxalyl chloride in the presence of copper toluenesulfonate at a temperature of 5 DEG C; then distilling a reaction product under a reduced pressure after the acylchloriration reaction; adding methylene dichloride; and distilling again under the reduced pressure. The preparation method for the cefbuperazone can increase a conversion rate of intermediate products and improve purity of a final product of the cefbuperazone.
Owner:珠海保税区丽珠合成制药有限公司 +1
Preparation method of cefbuperazone side chain
InactiveCN102838551AThe process steps are simpleSuitable for industrial productionOrganic chemistryBiochemical engineeringSide chain
The invention relates to a preparation method of a cefbuperazone side chain; and the cefbuperazone side chain is obtained by synthesizing two raw materials of D-threonine and DEPT through two-step reaction. According to the invention, reaction steps comprise cefbuperazone side chain synthesis and reaction liquid after treatment. According to the invention, in the after treatment process, crystallization of an extracting liquor uses a pH value controlling crystallization method, so that a concentrating link is omitted; and the invention is to provide a cefbuperazone immediate, which is prepared by simple technical steps and is suitable for industrial production.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
The preparation method of cefoperazone acid
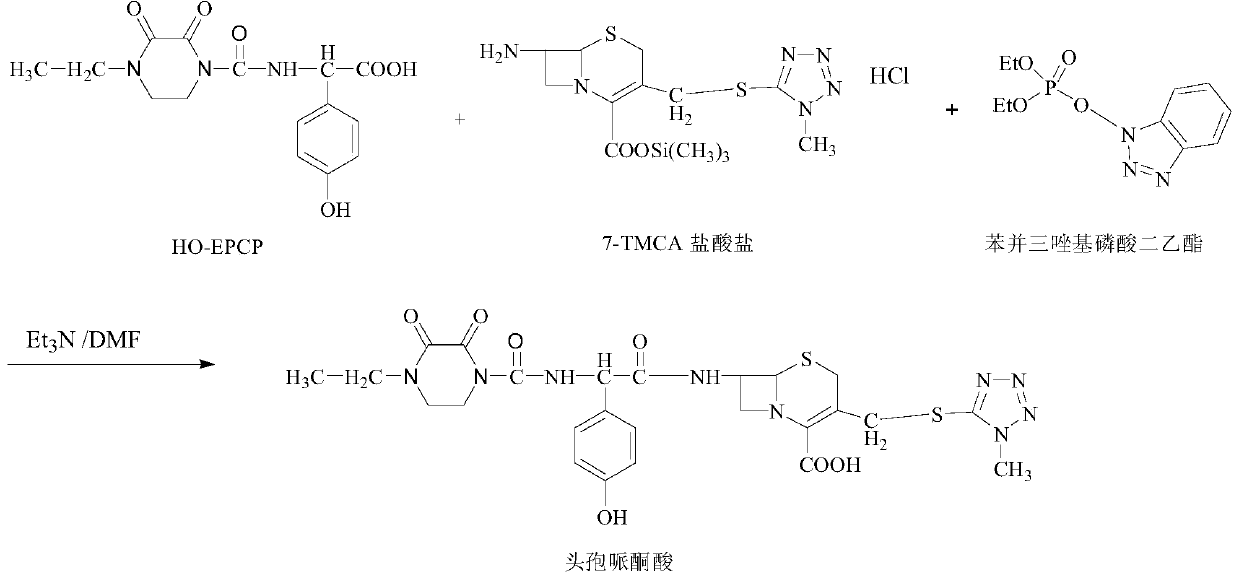
ActiveCN103641847BHigh purityThe reaction steps are simpleOrganic chemistryBulk chemical productionDiethyl phosphateThio-
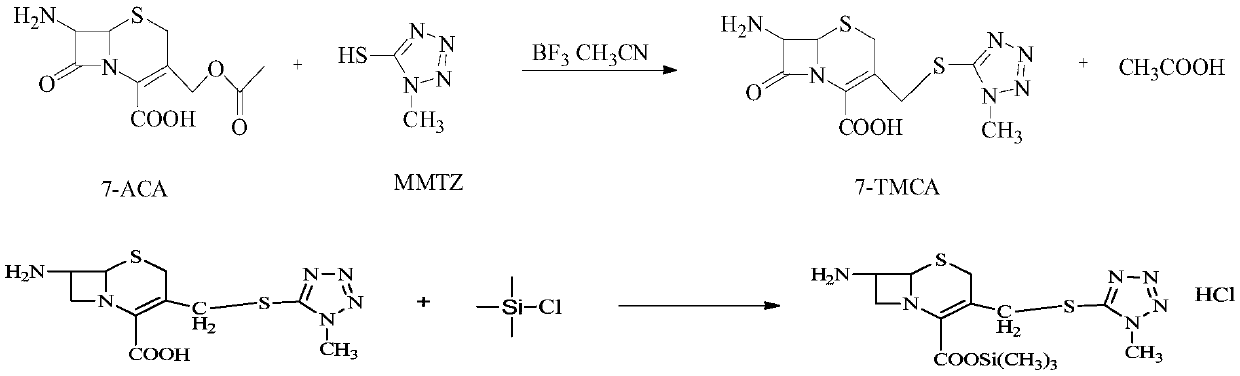
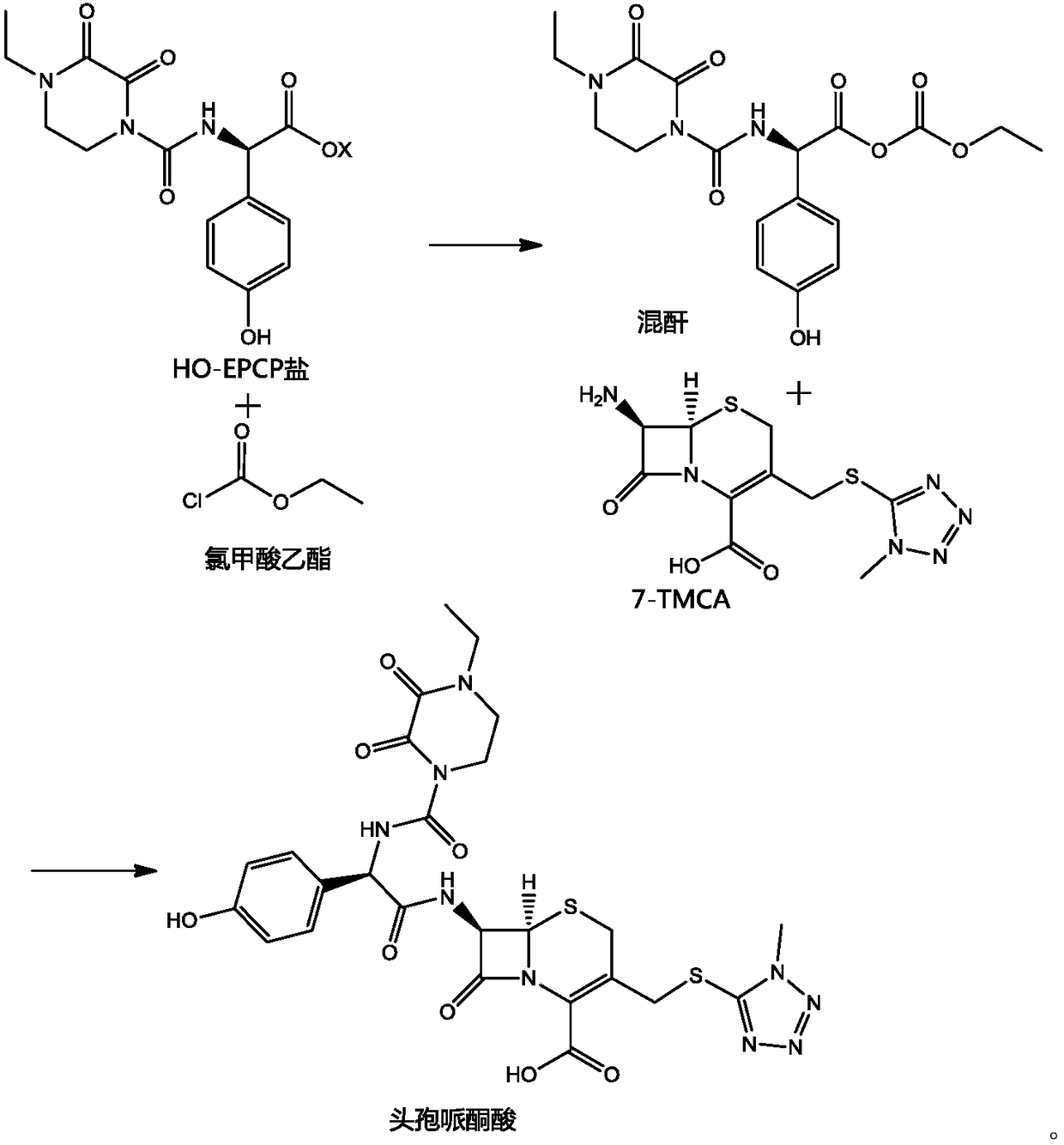
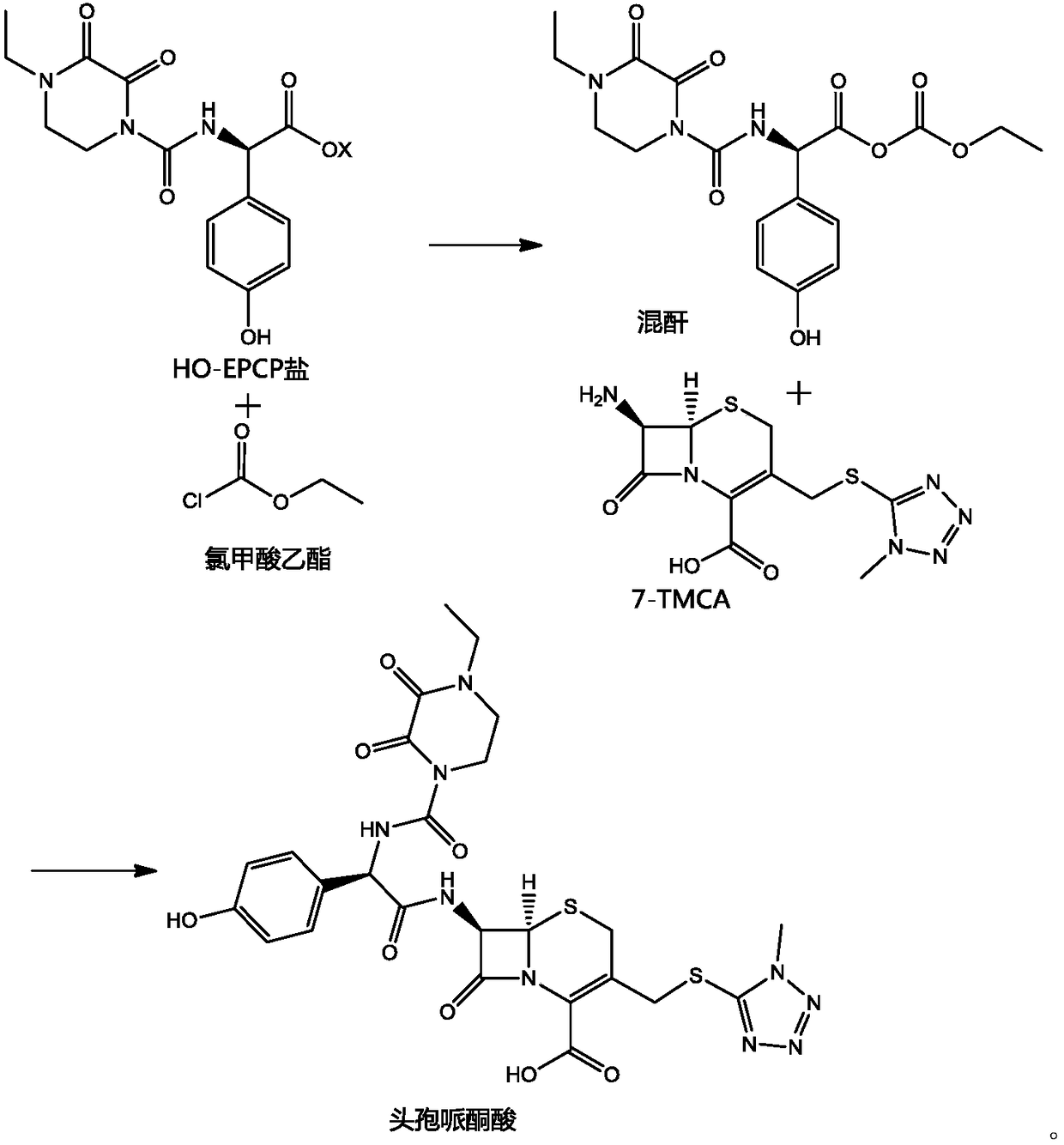
The invention belongs to the field of medicine, and particularly relates to a preparation method of cefoperazone acid. The method comprises the following steps: with 7-ACA (amin-oeephalosporanic acid) and 1-methyl-5-tetrazole-thione as raw materials under catalysis of boron trifluoride acetonitrile, reacting to prepare 7-TMCA (7-Amino-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-3-cephem-4-carboxylicacid)hydrochloride; carrying out carboxyl and amino group protection on the 7-TMCA hydrochloride by using trimethylchlorosilane; and with the group-protected 7-TMCA hydrochloride prepared in the step (1), HO-EPCP ((2R)-2-[(4-Ethyl-2,3-dioxopiperazinyl)carbonyllamino]-2-(4-hydroxyphenyl)acetic acid) and benzotriazole diethyl phosphonate as raw materials, carrying out N-acylation reaction in a DMF (Dimethyl Formamide) solution under catalysis of triethylamine, so as to obtain the cefoperazone acid, wherein acyl chloride is prepared by reaction in absence of the HO-EPCP, the benzotriazole diethyl phosphonate is directly added to directly carry out acylation reaction on the HO-EPCP and the group-protected 7-TMCA hydrochloride. Thus, the preparation method is simple in reaction step, low in cost and high in purity.
Owner:YIYUAN XINQUAN CHEM
A kind of synthetic method of cefoperazone acid
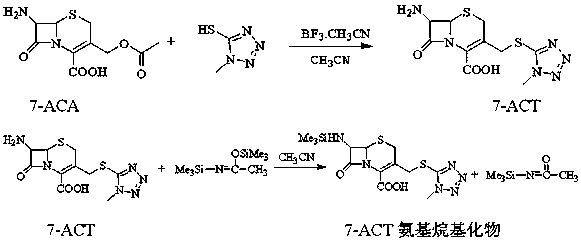
ActiveCN105566350BImprove conversion rateEasy to prepareOrganic chemistrySodium bicarbonateReaction temperature
The invention discloses a synthetic method of cefoperazone acid. The method comprises the steps of: reacting raw materials of 7-ACA and 1-methyl-5-mercapto tetrazole under the catalysis of boron trifluoride acetonitrile to obtain 7-ACT; dissolving the 7-ACT in a mixed solution of acetonitrile and N,O-(bis) trimethylsilyl acetamide to obtain a 7-ACT amino alkylate solution; mixing HO-EPCP, an organic solvent and a catalyst, adding phosphorus oxychloride for reaction at the temperature of -25 to -20 DEG C, wherein when the residue amount of HO-EPCP in the reaction solution is no more than 0.5%, the reaction is complete, so as to prepare a HO-EPCP acyl chloride solution; mixing and reacting 7-ACT amino alkylate solution with HO-EPCP acyl chloride solution, adding an aqueous solution of sodium bicarbonate after the reaction, standing foe stratification, filtering a lower layer material, adding water, crystallizing, filtering, washing and drying to obtain the cefoperazone acid. The method has the advantages of simpleness, high yield and stable intermediate.
Owner:HENAN KANGDA PHARMA
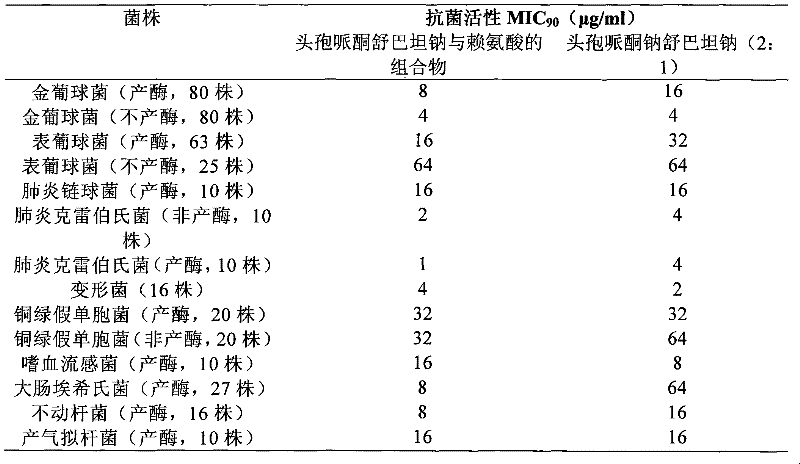
A kind of composition of cefoperazone sulbactam sodium and lysine
ActiveCN101780086BGood kidney safetyImprove stabilityAntibacterial agentsPowder deliveryMedicineInfective disorder
The invention belongs to the technical field of medicine, and specifically relates to a composition of cefoperazone sulbactam sodium and lysine, and also provides a preparation method of the composition injection, and is used for the treatment of cefoperazone drug-resistant patients. Use in medicine for infectious diseases caused by β-lactamase-producing bacteria sensitive to this product.
Owner:BEIJING SIHUAN PHARMA +1
Compound pharmaceutical composition of cefoperazone sodium and tazobactam sodium and preparation process thereof
ActiveCN104013629BImprove solubilityImprove stabilityAntibacterial agentsRespiratory disorderSolubilityAntioxidant
The invention discloses a composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium. The composite pharmaceutical composition is an injection, and is prepared from the following components in parts by weight: 3.5-4.5 parts of cefoperazone sodium, 1 part of tazobactam sodium, 0.5-1 part of ambroxol hydrochloride, 0-6 parts of excipient, 0-10 parts of isotonic agent, 0-1 part of antioxidant, a proper amount of citric acid-sodium citrate and 20 parts of injection water. The composite preparation of the cefoperazone sodium and the tazobactam sodium disclosed by the invention is stable in quality and significant in curative effect, not only can three active ingredients be evenly mixed, but also the composite pharmaceutical composition is excellent in stability, good in solubleness and good in clinical use safety.
Owner:福安药业集团庆余堂制药有限公司
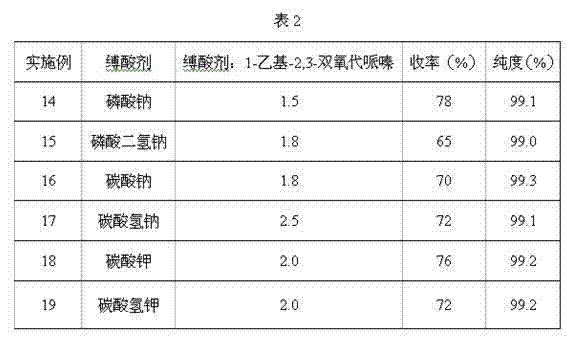
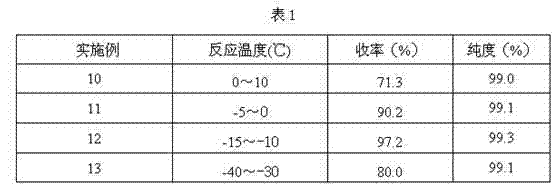
Synthetic method of cefbuperazone intermediate
ActiveCN106045925AResponse requirements are lowLow costOrganic chemistry methodsChemical synthesisRoom temperature
The invention discloses a synthetic method of a cefbuperazone intermediate, belonging to the technical field of chemical synthesis. The cefbuperazone intermediate is prepared by virtue of direct reaction of D-threonine and N-ethyl-2,3-dioxopiperazinyl formyl chloride in an alkali solvent at the room temperature. The synthetic method has the beneficial effects that the synthesis can be finished only through one step, has low reaction requirements and can be carried out at the room temperature; optimally, a reaction solvent is dichloromethane and can be taken as a later-period purification solvent, so that the cost is saved; the yield is high, and the product purity can reach 99.8%; and the operation is simple, the preparation is convenient, the cost is low, and the synthetic method is applicable to large-scale industrial production.
Owner:CHENGDU BAISHIXING SCI & TECH IND
Synthesis method of cefbuperazone
InactiveCN102250124BSimple designImprove responseOrganic chemistryBulk chemical productionTrimethylsilyl chlorideSilanes
Owner:HAINAN HERUI PHARMA +1
Refining method of cefbuperazone intermediate
ActiveCN101279981BSuitable for large-scale preparationEasy to operateOrganic chemistryAntibiotic YSodium salt
The invention relates to a refining method to prepare cefbuperazone intermediate compound with formulaI, aiming at providing a method to replace current column chromatography method to prepare cefbuperazone intermediate compound for economic and large-scale production. Cefbuperazone intermediate compound with formulaI is refined in isopropanol and highly pure product is obtained after separation process. Sodium salt of cefbuperazone is an antibiotic for cephamycins.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD +2
Preparation method of cefoperazone acid
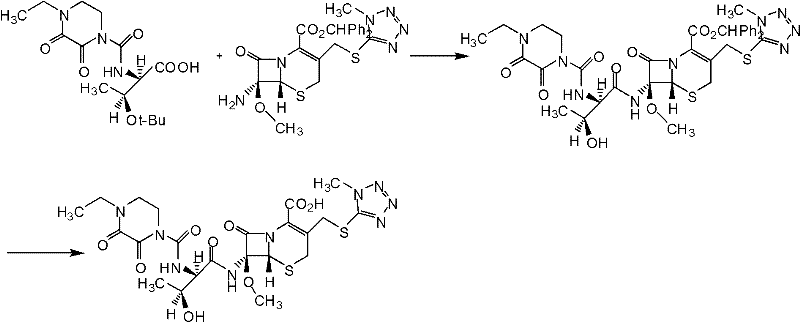
The invention relates to a preparation method of cefoperazone acid. The preparation method comprises the following steps: (1) in a dichloromethane system, allowing 7-TMCA to react with a silanizationreagent at 20-70 DEG C, and carrying out silanization protection on the amino group and the carboxyl group; (2) in a dichloromethane body and a DMA system, allowing HO-EPCP salt to react with ethyl chloroformate at the reaction temperature of 20-70 DEG C under the action of a catalyst to prepare mixed anhydride; (3) adding the 7-TMCA after silanization protection into the mixed anhydride, reactingat 20-70 DEG C to obtain a reaction solution; (4) hydrolyzing the reaction solution, adding alkali to adjust the pH to be 5.5-7.5, layering, adding solvent into the water phase, adding acid to adjustthe pH to be 1.5-2.5, crystalizing and filtering to obtain cefoperazone acid. The method disclosed by the invention has the advantages that the product yield can be improved, S-isomer can be reduced,the residual solvent is reduced, the solvent is easy to recycle, the wastewater does not contain phosphorus and is easy to process.
Owner:东瑞(南通)医药科技有限公司
Cefoperazone sodium composition freeze-dried powder for injection
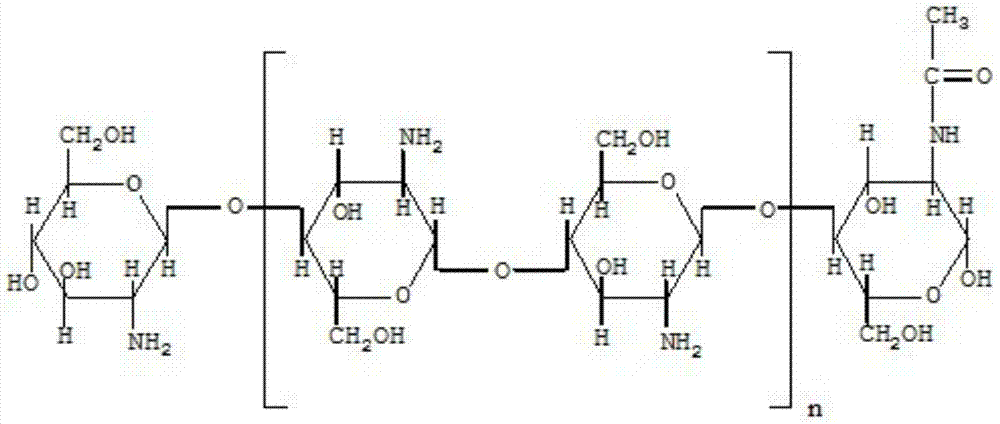
InactiveCN103536558AReduce the possibility of adverse reactionsHigh antibacterial activityAntibacterial agentsPowder deliveryChitosan nanoparticlesAdditive ingredient
The invention provides a cefoperazone sodium composition freeze-dried powder for injection, and belongs to the field of medicine and medicine preparation technology. The cefoperazone sodium composition freeze-dried powder comprises following raw material ingredients, by weight, 7.26 to 9.17 parts of cefoperazone sodium, 4.36 to 5.50 parts of chitosan nanoparticle, and 84.38 to 89.10 parts of injection water. Advantages of the cefoperazone sodium composition freeze-dried powder are that: 1) antibacterial activity of the cefoperazone sodium composition is increased significantly; 2) antibacterial spectrum is widened, and drug tolerance is reduced greatly; 3) improvement of activity is capable of shortening medication cycle of patients, and reducing occurrence likelihood of adverse reaction caused by accumulation of cefoperazone sodium; and 4) the chitosan nanoparticle can be used as a freeze-dried skeleton agent of the freeze-dried powder injection instead of mannitol, so that active effects of mannitol on human bodies are avoided.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Preparation method for cefbuperazone
The invention provides a preparation method for cefbuperazone. The preparation method comprises the steps of performing an acylchloriration reaction on D-alpha-(4-ethyl-2 ,3-dioxo-piperazine-carboxamido)-beta-(S) hydroxybutyric acid and oxalyl chloride in the presence of copper toluenesulfonate at a temperature of 5 DEG C; then distilling a reaction product under a reduced pressure after the acylchloriration reaction; adding methylene dichloride; and distilling again under the reduced pressure. The preparation method for the cefbuperazone can increase a conversion rate of intermediate products and improve purity of a final product of the cefbuperazone.
Owner:珠海保税区丽珠合成制药有限公司 +1
A kind of cefbuperazone pharmaceutical composition and preparation method thereof
InactiveCN103585110BEasy to stratifyFast dissolutionAntibacterial agentsPowder deliveryHigh resistanceDissolution
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Cefbuperazone pharmaceutical composition and preparation method thereof
InactiveCN103585110ASolve for uniformitySolve the problem of easy delamination during storage and transportationAntibacterial agentsOrganic active ingredientsBottlePowder injection
A cefbuperazone pharmaceutical composition has the advantages of high uniformity, strong anti-layering performance, fast dissolving rate and good fluidity. A product obtained by packaging is accord with pharmacopeia index requirements about powder injection bottle weight difference, solution clarity and the like.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Medicinal composition of cefoperazone sodium and tazobactam sodium
ActiveCN102274233BImprove stabilityQuality is easy to controlAntibacterial agentsHeterocyclic compound active ingredientsPowder diffractionSubstance content
Owner:江西璟瑞药业有限公司
A kind of cefoperazone sodium sulbactam sodium composition
ActiveCN105853441BImprove antibacterial propertiesReduce body accumulationAntibacterial agentsPharmaceutical non-active ingredientsSulbactam SodiumCefbuperazone
The invention provides a cefoperazone sodium and sulbactam sodium composition, which comprises the following raw materials in parts by weight: 9-10 parts of cefoperazone sodium, 1 part of sulbactam sodium and 0.05-0.07 part of glutathione. The glutathione is added to the composition, so that the composition has relatively good antibacterial effect. Furthermore, a little of sulbactam sodium is utilized, so that accumulation of the sulbactam sodium in a body is reduced; and the production cost is reduced. In addition, by a novel sulbactam sodium crystal, the composition provided by the invention has relatively good stability.
Owner:HAINAN HERUI PHARMA
Stable cefoperazone potassium clavulanatein medicine compound preparation
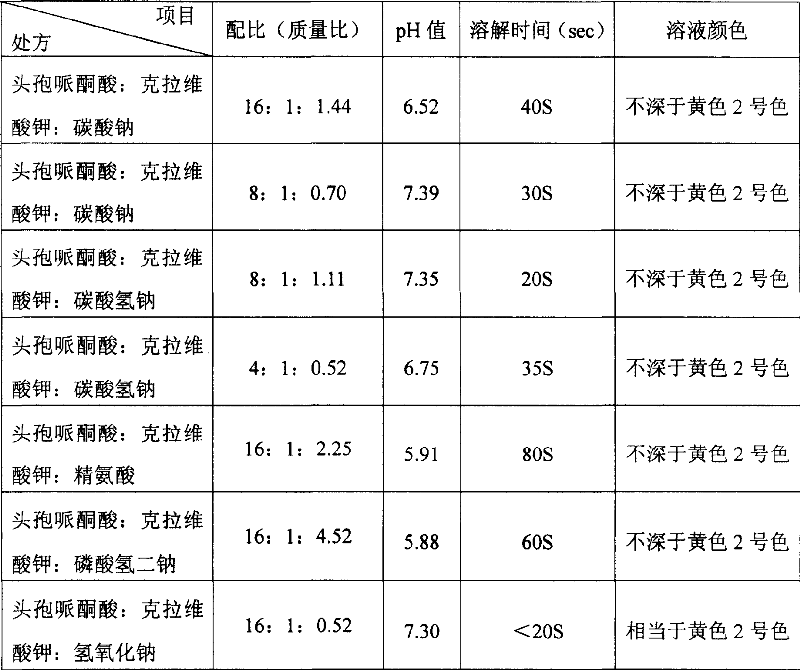
The invention discloses a stable compound preparation of cefoperazone-piperacillin clavulanate drug, which is comprised by cefoperazone acid, piperacillin clavulanate and latent solvent, which weight ratio is 16~1:1:8.8~0.06. The latent solvent is preferred selected from sodium carbonate and sodium bicarbonate. Related substances content of the compound preparation, labelled content of the cefoperazone acid and the piperacillin clavulanate in the invention do not change much in influencing factor test, long-term sample tests and accelerated test in 40 DEG C. which accord with the standard of pharmacopoeia with stable product quality.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate
ActiveCN101921237BReduce generationReduce separation proceduresOrganic chemistryEthyl chloroformateOrganic solvent
The invention discloses a method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate. In the method, 1-ethyl-2,3-dioxypiperazine and chloro-formate are used as raw materials and reacted in an organic solvent system in the presence of an acid binding agent to form 4-ethyl-2,3-dioxypiperazine-1-formate, wherein the molar ratio of the 1-ethyl-2,3-dioxypiperazine to the acid binding agent to the chloro-formate is 1:1.0-3.0:1.0-2.0; the chloro-formate is methyl chloroformate or ethyl chloroformate; and the 4-ethyl-2,3-dioxypiperazine-1-formate is 4-ethyl-2,3-dioxypiperazine-1-methyl formate or 4-ethyl-2,3-dioxypiperazine-1-ethyl formate. The method greatly reduces cost, simplifies process, reduces byproducts, improves product purity and reduces solvent separation processes; and the prepared product can be used as an intermediate for piperacillin and cefoperazone and is suitable for industrial production.
Owner:山东艾孚特科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com