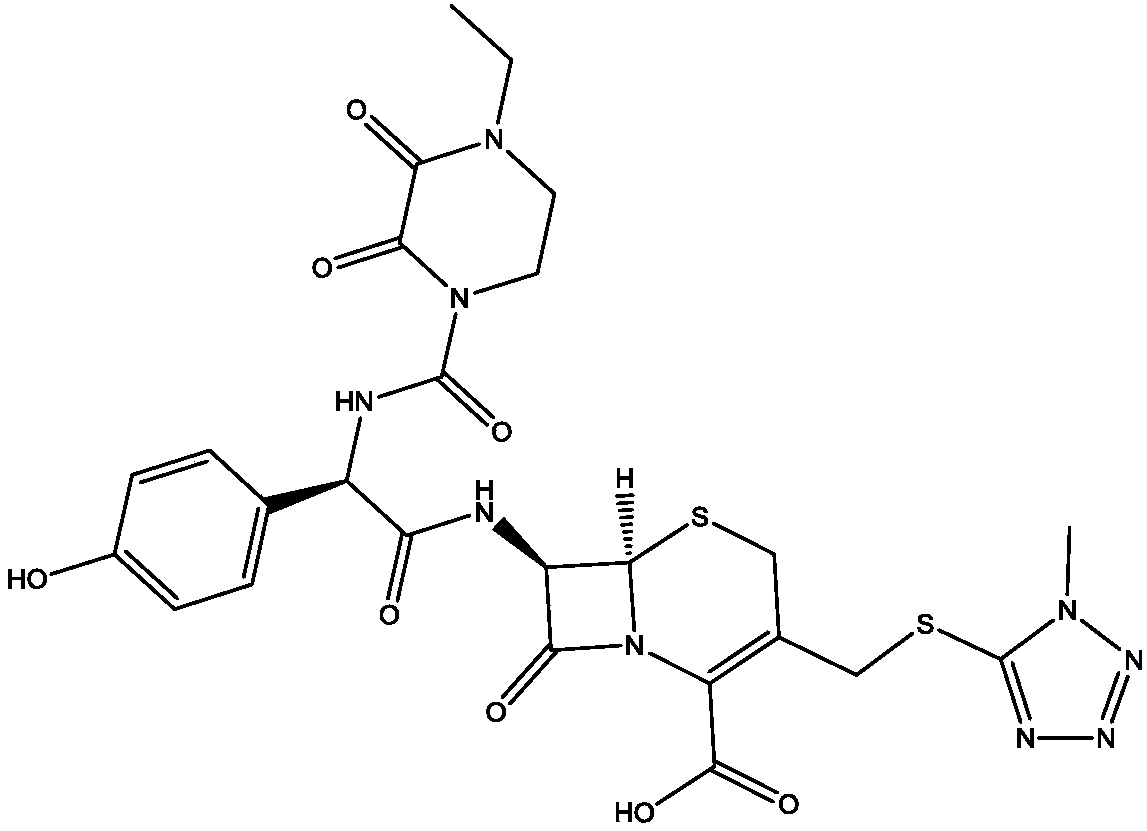
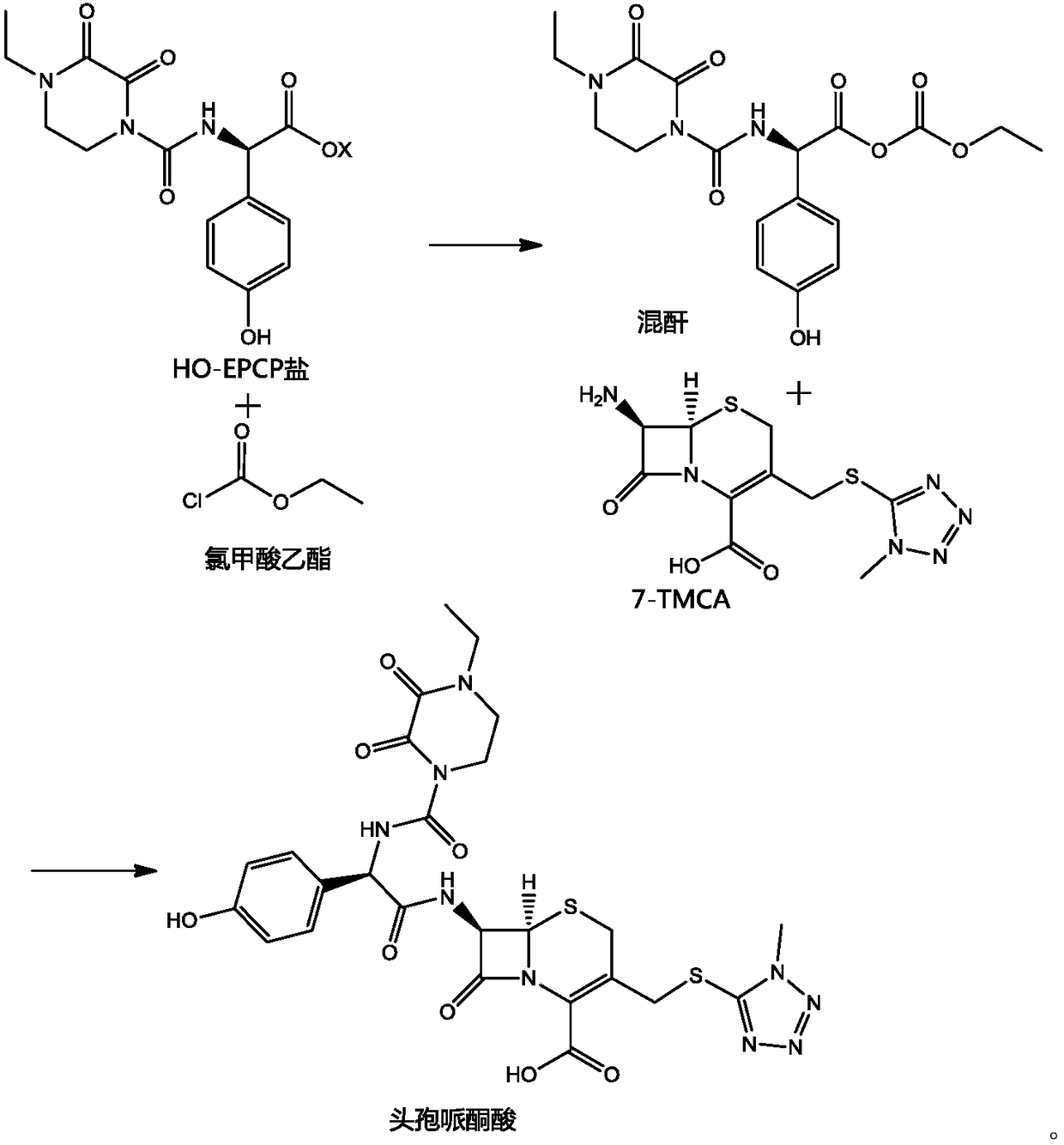
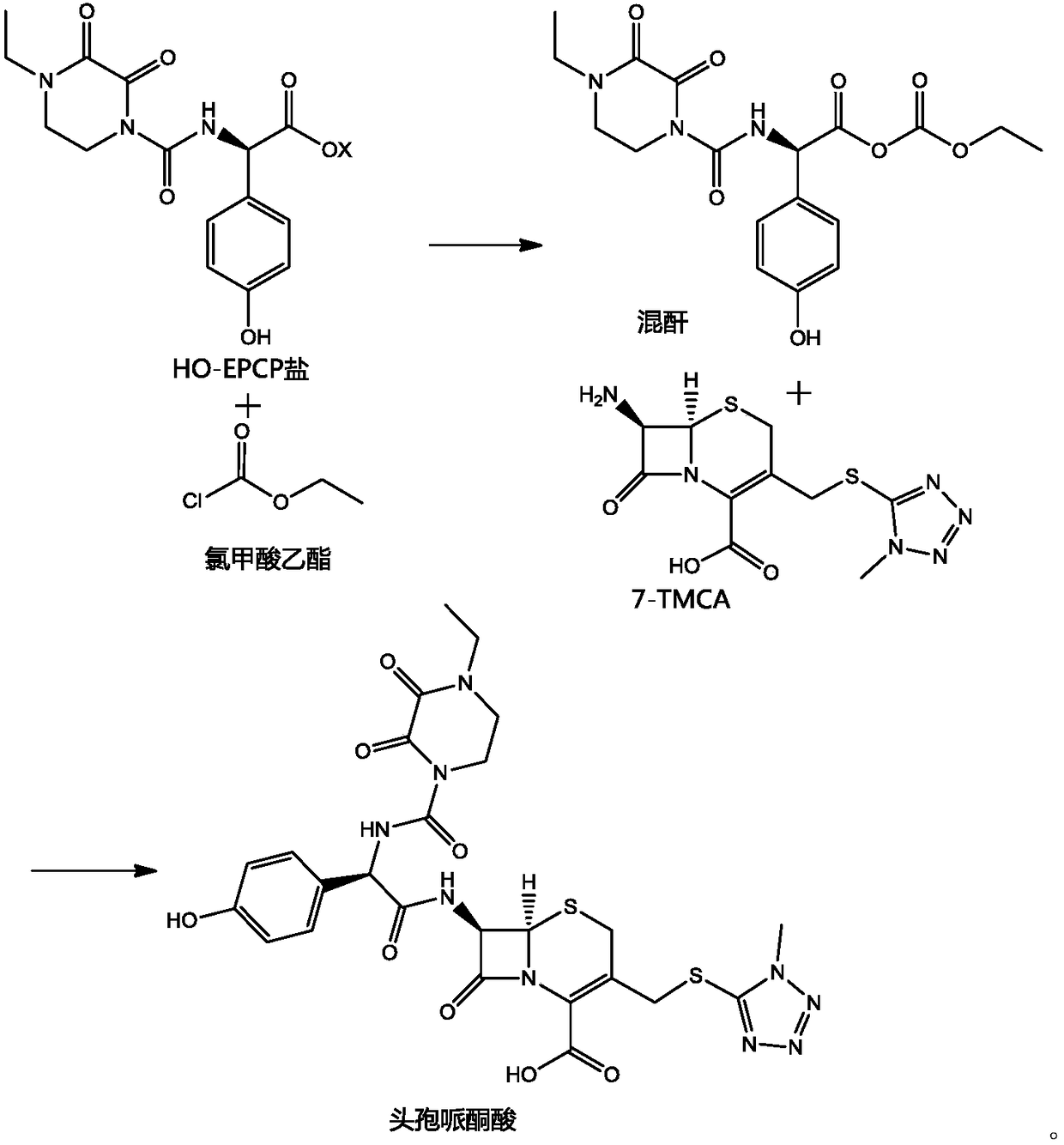
Preparation method of cefoperazone acid
A technology of cefoperazone acid and dichloromethane, which is applied in the field of preparation of cefoperazone acid, can solve the problems of high residual solvent in the product, poor product fluidity and high S-isomer, and achieves difficult to encapsulate impurities and low fluidity. , the effect of low S-isomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Add 20g of 7-TMCA to 40mL of dichloromethane, add 7.86g of hexamethyldisilazane, 5.29g of trimethylchlorosilane, react at 45°C for 4 hours, cool and set aside.
[0026] (2) 80mL of dichloromethane and 20mL of DMA, add 22.46g of HO-EPCP, dropwise add 6.85g of triethylamine, cool down to -50°C, add 0.04g of pyridine, dropwise add 7.95g of ethyl chloroformate, and react for 2h.
[0027] (3) Add (1) into (2), control the temperature at -40~-45°C, and react for 2 hours.
[0028] (4) After the reaction, add 120 mL of water, adjust the pH to 6.5 with triethylamine, add 20 mL of acetone, adjust the pH to 2.0 with acid to crystallize, filter and dry to obtain 35.33 g of cefoperazone acid, and the weight yield is 176.65%.
Embodiment 2
[0030] (1) Add 20g of 7-TMCA to 40mL of dichloromethane, add 18g of N,O-(bis)trimethylsilylacetamide, react at 35°C for 1 hour, cool down, and set aside.
[0031] (2) 80mL of dichloromethane and 20mL of DMA, add 22.85g of HO-EPCP sodium salt, cool to -40°C, add 0.05g of lutidine, dropwise add 8.3g of ethyl chloroformate, and react for 2h.
[0032] (3) Add (1) into (2), control the temperature at -35~-40°C, and react for 2 hours.
[0033] (4) After the reaction, add 120 mL of water, adjust the pH to 7.0 with sodium carbonate, add 20 mL of acetonitrile, adjust the pH to 1.8 with acid to crystallize, filter and dry to obtain 35.40 g of cefoperazone acid, and the weight yield is 177%.
Embodiment 3
[0035] (1) Add 20g of 7-TMCA to 40mL of dichloromethane, add 13.76g of hexamethyldisilazane, react at 35°C for 1 hour, cool down, and set aside.
[0036] (2) 80mL of dichloromethane and 20mL of DMA, add 22.85g of HO-EPCP potassium salt, cool down to -50°C, add 0.06g of N-methylmorpholine, dropwise add 7.3g of ethyl chloroformate, and react for 2h.
[0037] (3) Add (1) into (2), control the temperature at -45~-50°C, and react for 2 hours.
[0038] (4) After the reaction, add 120 mL of water, adjust the pH to 6.0 with sodium bicarbonate, add 20 mL of methanol, adjust the pH to 2.2 with acid to crystallize, filter and dry to obtain 35.10 g of cefoperazone acid, and the weight yield is 175.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com