Preparation method for cefbuperazone
A technology of cefrazone and ethyl, applied in the field of preparation of cefrazone, can solve the problems of high cost, slow reaction efficiency, no crystals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The preparation of embodiment 1 cefbuperazone xylene ester
[0076]
[0077] Under the protection of nitrogen, into a 250 ml three-necked flask, sequentially add 20 gram, 100 milliliters of dichloromethane, 10 grams of copper tosylate, stirring and dissolving, cooling in an ice-water bath to 0 ℃ < T < 5 ℃, adding dropwise dichloromethane (10 milliliters) of pre-prepared oxalyl chloride (7 milliliters) solution; after dropping, continue to control the temperature T<5°C, add dropwise a solution of DMF (3 ml) in dichloromethane (10 ml) prepared in advance; after dropping, stir and react at 0-5°C;
[0078] After the reaction is completed, under the conditions of temperature 30-35°C and vacuum degree -0.09-0.1MPa, distill off DMF and dichloromethane under reduced pressure, and distill off unreacted oxalyl chloride at the same time until there is no distillate, leaving Add 50 ml of dichloromethane to the viscous oil, dissolve it, and distill under reduced pressure at a ...
Embodiment 2
[0091] The preparation of embodiment 2 cefbuperazone crude product
[0092] Into a 250 ml reactor, 35 g of cefbuperazone diphenylmethyl ester and 60 ml of anisole were sequentially added, stirred to dissolve and cool down; then 70 ml of trifluoroacetic acid was added dropwise to the reaction system. After dropping, stir and react at room temperature for 1.5 hours; then transfer the reaction solution to a 1-liter container, and add 150 milliliters of ethyl acetate to it, stir at room temperature, add 450 milliliters of petroleum ether while stirring, separate out solid, and stir at room temperature; Filter to obtain light yellow solid filter cake;
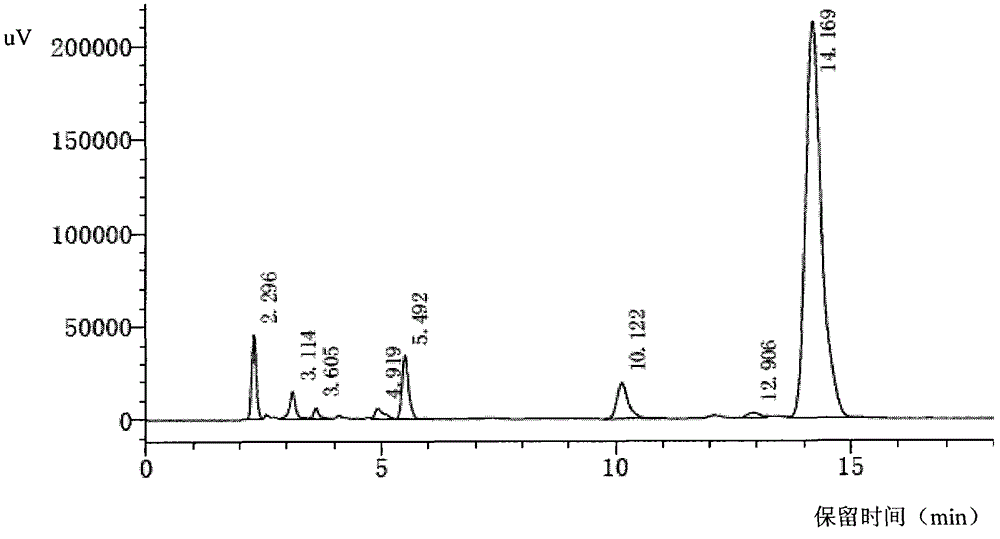
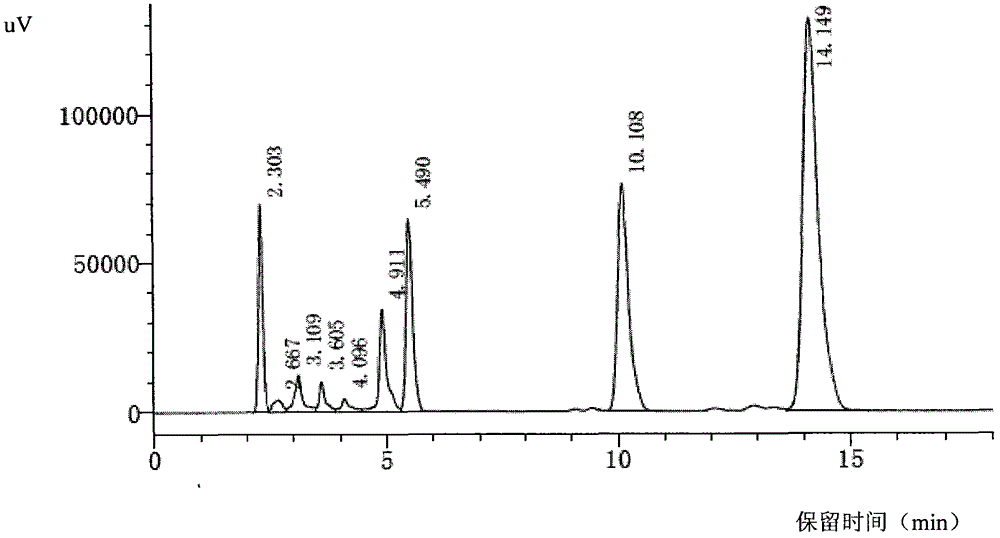
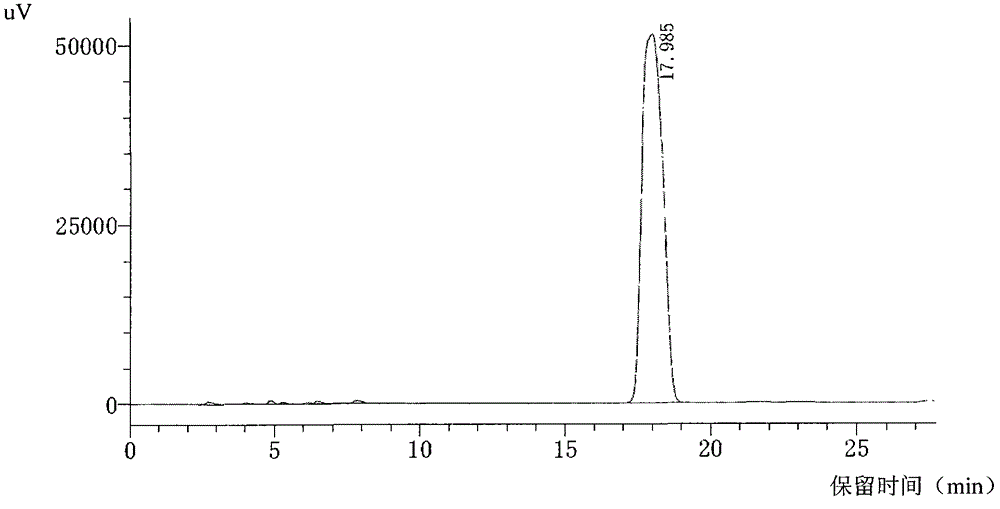
[0093] The filter cake was added to 200 ml of acetone, stirred in an ice-water bath; then filtered with suction to obtain a light yellow solid filter cake, which was vacuum-dried at 40° C. to constant weight to obtain about 13 grams of light yellow solid powder. The conditions described in Example 4 were used to detect by HPLC, a...
Embodiment 3
[0094] The purification of embodiment 3 cefbuperazone
[0095] In 5 liters of reactors, add cefbuperazone crude product (40 grams), dichloromethane (580 milliliters), stir at room temperature; Then slowly add methanol (330 milliliters), dissolve, then add gac (5 grams), stir at room temperature and decolorize The reaction system is suction filtered to obtain a light yellow filtrate; the filtrate is concentrated under reduced pressure to remove about 3 / 4 of the solvent (even if the volume of the entire reaction system is reduced by 3 / 4), to obtain a residual solution; add acetone (400 milliliters) , stirred at room temperature, and a light yellow solid was precipitated; the solid obtained was filtered by suction, and the filter cake was vacuum-dried at 60° C. to constant weight to obtain about 35 grams of a light yellow to yellow-white solid powder.
[0096] According to the quality standard of cefbuperazone sodium on the 429th page of the 15th edition of Japanese Pharmacopoe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com