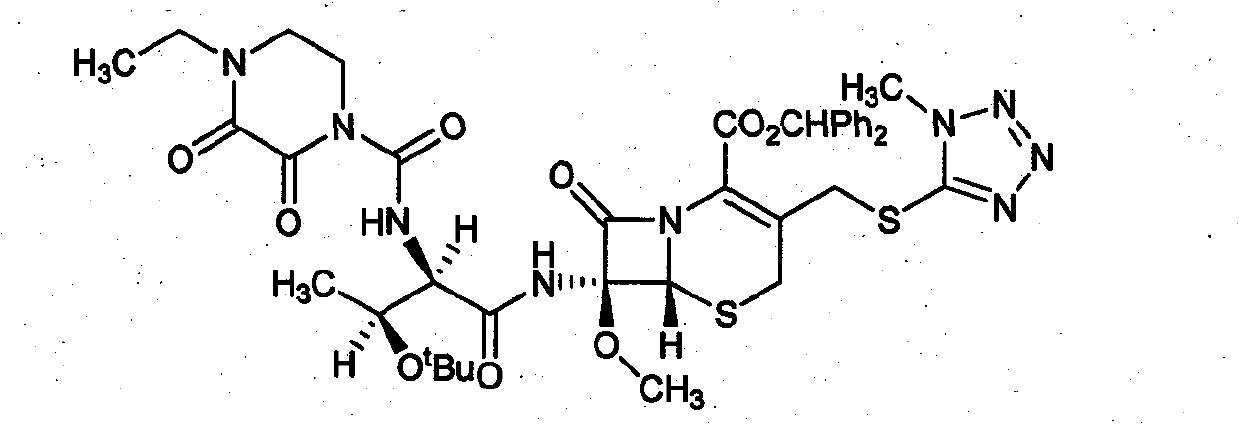
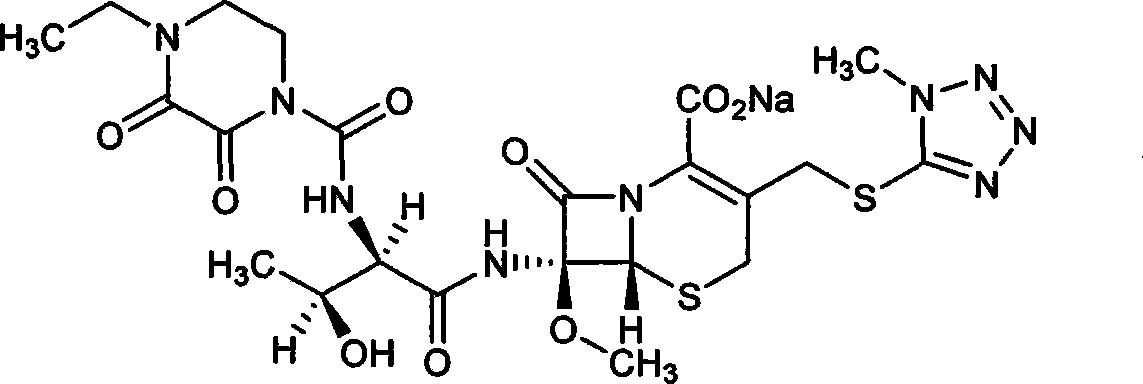
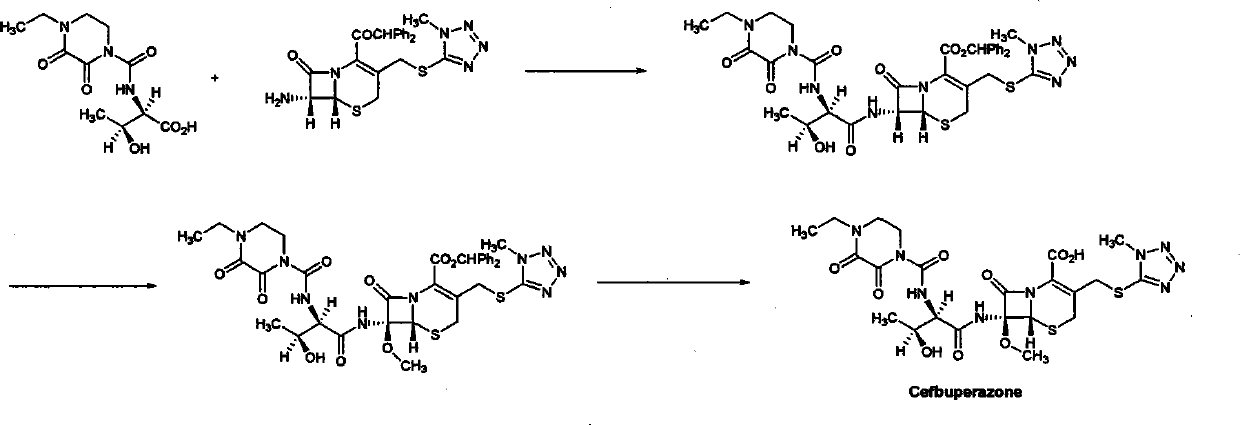
Refining method of cefbuperazone intermediate
A refining method and a technology for intermediates, applied in the field of preparation of cefbuperazone intermediates, can solve the problems of increased preparation costs, high requirements for product preparation scale, increased large-scale production, etc., and meet the requirements of equipment and preparation conditions. High, high preparation purity, simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 formula I intermediate
[0036] According to the method described in Example 4 of the patent "GB2048241": 200 grams of cefbuperazone's 7-position side chain was dissolved in 700 milliliters of anhydrous methylene chloride, and under ice-cooling, 100 grams of oxalyl chloride and 30 milliliters of anhydrous "N , N-dimethylformamide", reacted at room temperature for 30 minutes. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, 800 ml of anhydrous dichloromethane was added to the residue, the temperature was lowered to -50°C under nitrogen flow, and 300 g of "7-MAC" and 100 g of dimethylaniline were added in batches. Stir overnight at -20°C. The solvent was evaporated to dryness under reduced pressure, 1000 ml of water and 2000 ml of ethyl acetate were added to the residue, the organic phase was separated, washed with water, and the solvent was evaporated to dryness under reduced pressure to obta...
Embodiment 2
[0037] The refining of embodiment 2 formula I intermediate
[0038] Add 100 g of the crude intermediate of formula I prepared according to Example 1 to 800 ml of isopropanol, stir vigorously at 20-25° C. for 2 hours, and then keep it overnight. After filtration, the filter cake was vacuum-dried at room temperature to obtain 82 grams of light yellow powdery solid, HPLC purity: 95.6%.
Embodiment 3
[0039] The refining of embodiment 3 formula I intermediate
[0040] Add 50 g of the crude intermediate of formula I prepared according to Example 1 to 200 ml of isopropanol, stir vigorously at 20-25° C. for 2 hours, and then keep it overnight. After filtration, the filter cake was vacuum-dried at room temperature to obtain 45 g of light yellow powdery solid, HPLC purity: 93.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com