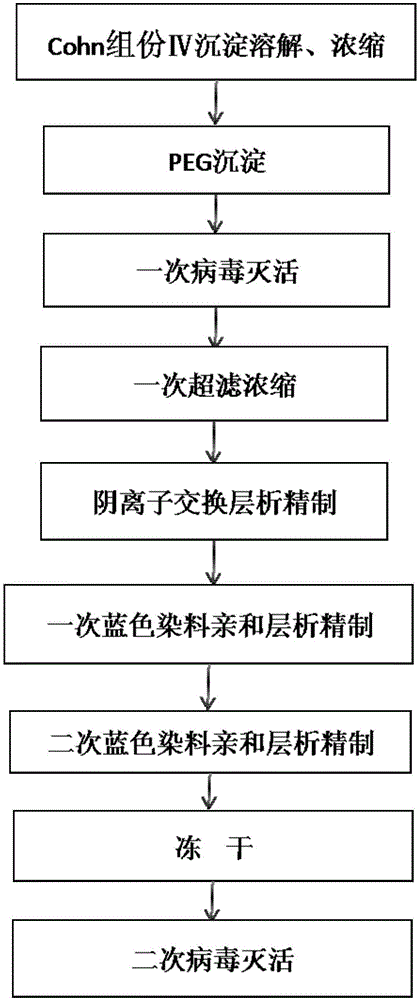
Method for purifying alpha1-antitrypsin from Cohn component IV precipitate
An anti-trypsin and albumin technology, which is applied in the field of protein purification, can solve the problems of unsuitable process production and cumbersome preparation methods, and achieve the effects of simplifying the production process, reducing ultrafiltration and concentration steps, and ensuring the safety of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
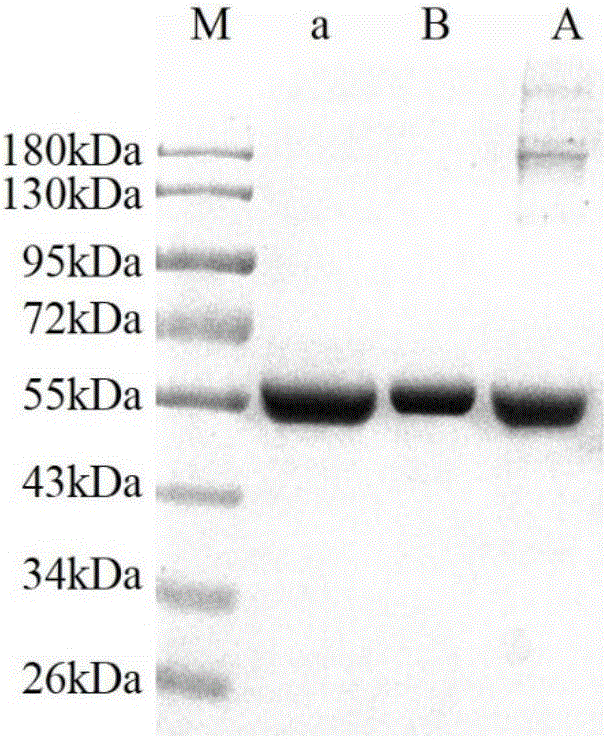
[0058] a) Dissolving and concentrating Cohn component IV precipitate: 200g Cohn component IV precipitate was dissolved in 2000ml water, stirred at 4°C for 3h, adjusted to pH 7.5, and centrifuged to remove diatomaceous earth to obtain Cohn component IV solution (electrophoretic diagram See figure 2 ). The solution of Cohn component IV was concentrated by ultrafiltration to 4 times the volume to 500ml to obtain the concentrated solution of Cohn component IV with a protein content of 20-22mg / ml.
[0059] b), PEG precipitation: adjust the pH value of the Cohn component IV concentrate to 5.0, add 50 g of PEG4000 while stirring at 4°C to a final concentration of 10% (w / v, g / 100ml), and continue stirring for 30 -45min to fully dissolve PEG4000; stand at 4°C for 30-45min, centrifuge at 6000g for 20min, discard the precipitate, and take the supernatant; the precipitate includes macromolecular (molecular weight > 180kDa) miscellaneous proteins, transferrin, albumin and vitamin D bind...
Embodiment 2
[0073] a) Dissolving and concentrating Cohn component Ⅳ precipitation: 1.2kg Cohn component Ⅳ precipitation was dissolved in 10.8L water, stirred at 0°C for 4h, adjusted to pH 7.8 with 0.5M NaOH, and used a filter press for solid-liquid separation to remove silicon Celite to obtain Cohn component IV solution. The solution of Cohn component IV was concentrated by ultrafiltration to 7 times volume to 2L to obtain the concentrated solution of Cohn component IV with a protein content of 29-31 mg / ml.
[0074] b), PEG precipitation: Cohn component IV concentrate was adjusted to pH 5.2 with 0.5M HCl, and 300g PEG 4000 was added while stirring at 0°C to a final concentration of 15% (w / v, g / 100ml). Continue to stir for 30-45min to fully dissolve PEG4000 after completion; stand at 0°C for 30-45min, centrifuge at 4000g for 30min, discard the precipitate, and take the supernatant.
[0075] c), one-time virus inactivation: S / D method virus inactivation, the supernatant is adjusted to pH 7.0...
Embodiment 3
[0087] a) Dissolving and concentrating Cohn component Ⅳ precipitate: 4kg Cohn component Ⅳ precipitate was dissolved in 32L water, stirred at 10°C for 2h, adjusted to pH 8.0 with 0.5M NaOH, and removed diatomaceous earth by solid-liquid separation using a filter press , to obtain Cohn component IV solution. The solution of Cohn fraction IV was concentrated by ultrafiltration to 10 times volume to 3.6 L to obtain the concentrated solution of Cohn fraction IV with a protein content of 38-40 mg / ml.
[0088] b), PEG precipitation: the Cohn component IV concentrate was adjusted to pH 5.5 with 0.5M HCl, and 432g PEG 4000 was added while stirring at 10°C to a final concentration of 12% (w / v, g / 100ml). Continue to stir for 3-4 hours to fully dissolve PEG 4000; stand overnight at 10°C, centrifuge at 4000g for 60 minutes, discard the precipitate, and take the supernatant.
[0089] c), one-time virus inactivation: S / D method virus inactivation, the supernatant is adjusted to pH 7.5 with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com