Preparation method of cefoperazone acid
A technology of cefoperazone acid and cefoperazone sodium, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as difficult product quality assurance, complex synthesis reaction routes, and high raw material costs, and achieves no by-product formation, simple preparation process, and high preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A preparation method of cefoperazone acid comprises the following steps: cefoperazone sodium is used as a raw material, a precipitating agent is added to a cefoperazone sodium solution to decolorize, and a dispersant is added to the decolorization solution for crystallization to obtain cefoperazone acid.
[0034] The quality index of cefoperazone sodium meets any one or a combination of total impurities > 3.0%, maximum single impurities > 2.0% or impurity A > 3.0%.
[0035] The preparation method comprises the following steps:
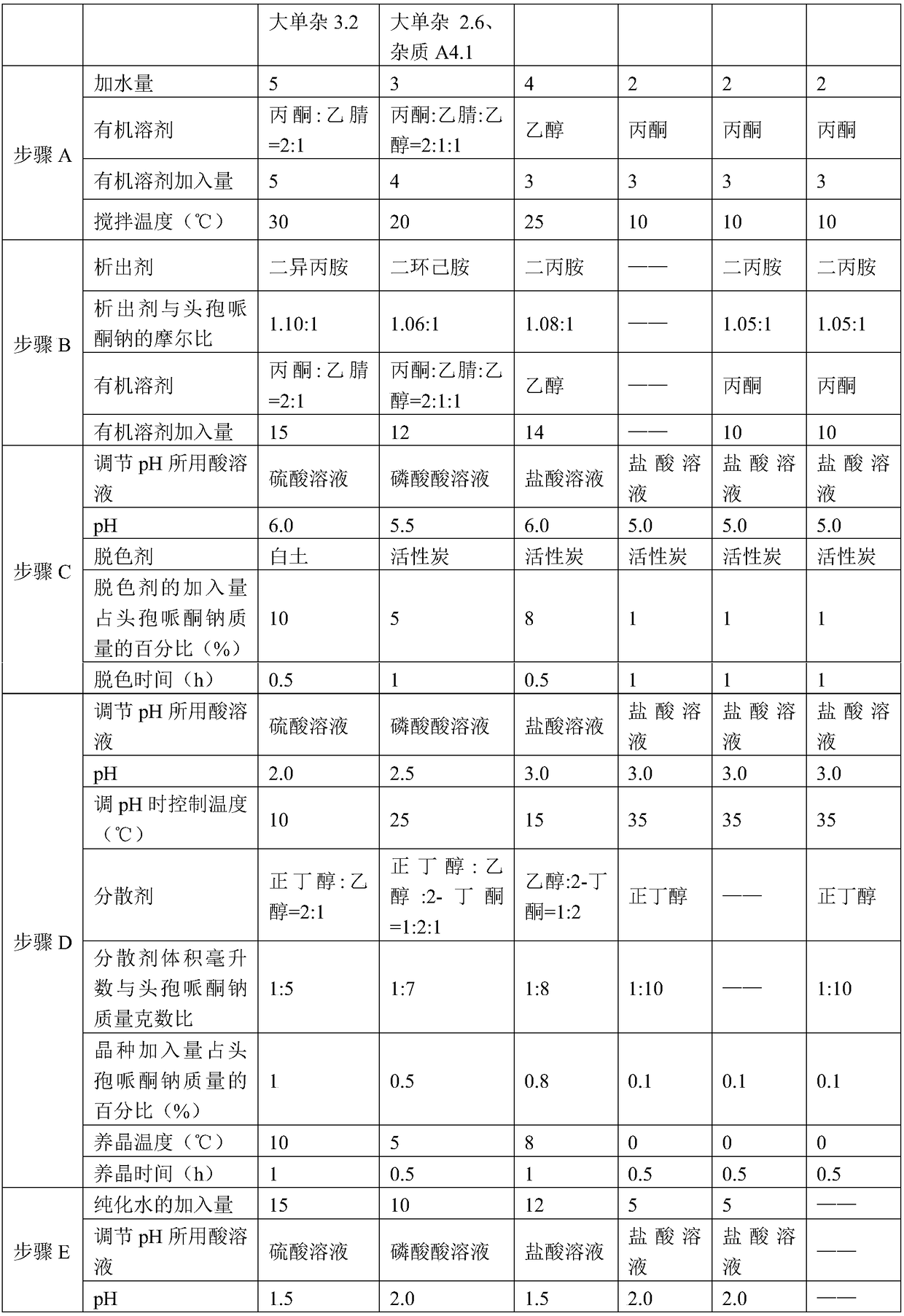
[0036] A. Add 2 to 5 times the quality of cefoperazone sodium in water to dissolve cefoperazone sodium, then add an organic solvent with 3 to 5 times the quality of cefoperazone sodium, stir and dissolve, and control the temperature during stirring to be 10 to 30°C to obtain a cefoperazone sodium solution;
[0037] The organic solvent is any one or a combination of acetone, acetonitrile or ethanol;
[0038] B. in the cefoperazone sodium solution,...
Embodiment 1
[0047] A preparation method of cefoperazone acid comprises the following steps: cefoperazone sodium is used as a raw material, a precipitating agent is added to a cefoperazone sodium solution to decolorize, and a dispersant is added to the decolorization solution for crystallization to obtain cefoperazone acid.
[0048] The preparation method comprises the following steps:
[0049] A. in 40g cefoperazone sodium (cefoperazone sodium total miscellaneous is 4.2%), add the water dissolving of 80mL, then add the acetone of 120mL, control stirring temperature is 10 ℃, stirring and dissolving is clear to dissolving, obtains cefoperazone sodium solution;
[0050] B. Add dropwise dipropylamine 8.62mL in the cefoperazone sodium solution, then add acetone 400mL, filter to obtain the precipitate;
[0051] C. The precipitate was adjusted to pH 5.0 with hydrochloric acid solution, and 0.4 g of activated carbon was added to decolorize for 1 hour. The filtrate was collected after decolorizing...
Embodiment 2~4
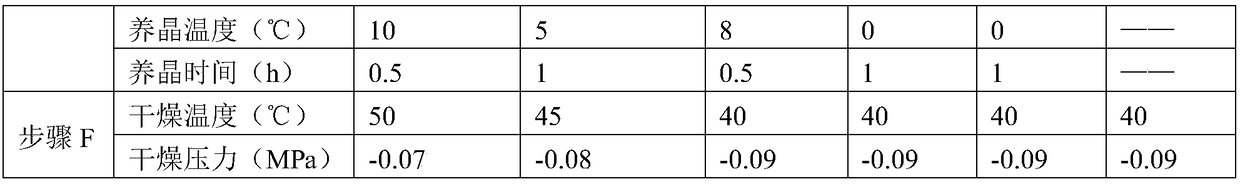
[0056] Embodiments 2 to 4 are identical to the production process steps in Example 1, and the difference is the selection of process parameters, as shown in Table 1 below, wherein "water addition" is the addition of milliliters of water to the mass grams of cefoperazone sodium How many times; "organic solvent addition" is how many times the milliliters of organic solvent is the mass grams of cefoperazone sodium; "the addition of dispersant" is how many times the milliliters of dispersant is the mass grams of cefoperazone sodium; "The amount of water added" is how many times the milliliters of purified water is the mass grams of cefoperazone sodium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com