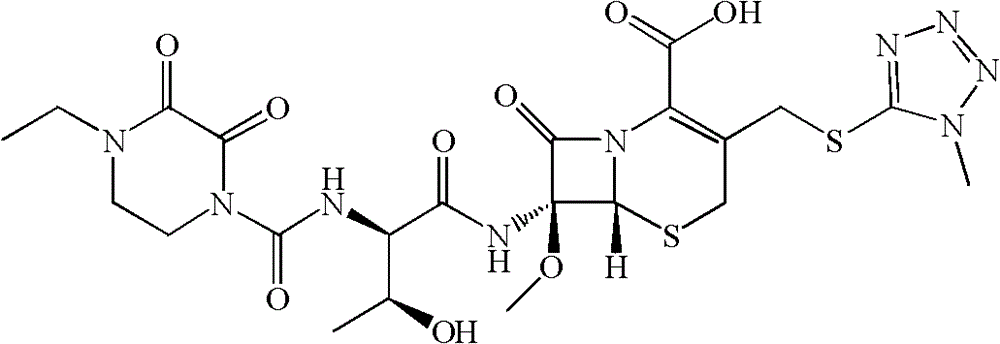
Synthesis method of cefbuperazone
A synthetic method, the technology of cefbuperazone, applied in the field of medicine, can solve the problems of increasing the difficulty of large-scale production, aggravating environmental hazards, and high requirements for preparation scale, and achieves easy operation and control, easy realization, and simple synthetic route design reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
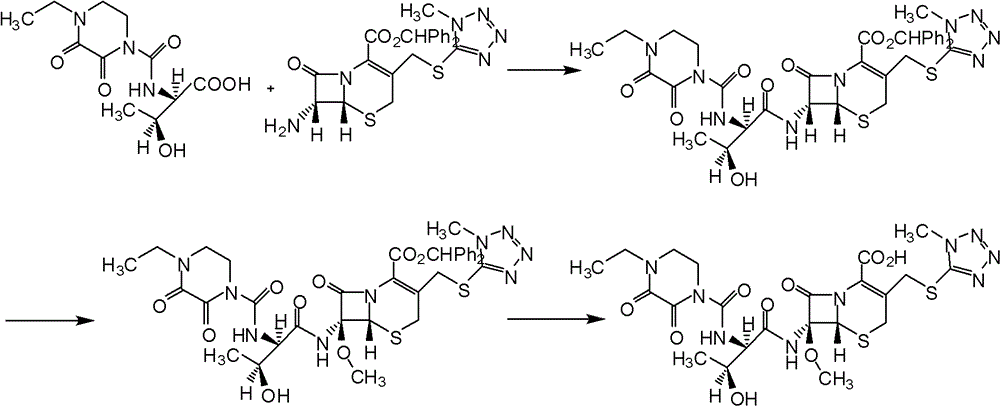
Embodiment 1
[0049] (1) Preparation of 7-MAC silyl compound
[0050]
[0051] 7-MAC 7-MAC silyl compound
[0052] Add 1.05kg of 7-MAC, 6.4kg of dichloromethane and 0.22kg of trimethylchlorosilane into the reaction tank, control the temperature at 20-25°C and stir for 40min, then cool down to below -5°C for use.
[0053] (2) preparation of cefbuperazone side chain acid chloride
[0054]
[0055] Cefbuperazone Side Chain Cefbuperazone Side Chain Acid Chloride
[0056] Add 0.61kg of cefbuperazone side chain, 0.19kg of tetrahydrofuran and 5.3kg of dichloromethane into the reaction tank. After stirring and dissolving, cool down to -30~-25°C and slowly add 0.33kg of phosphorus oxychloride dropwise. During the process, the temperature was controlled not to exceed -20°C, and the dripping was completed within 30 minutes, and the reaction was stirred for another 30 minutes.
[0057] (3) preparation of cefbuperazone diphenylmethyl ester
[0058]
[0059] Cefbuperazone Side Chain Acid Chlor...
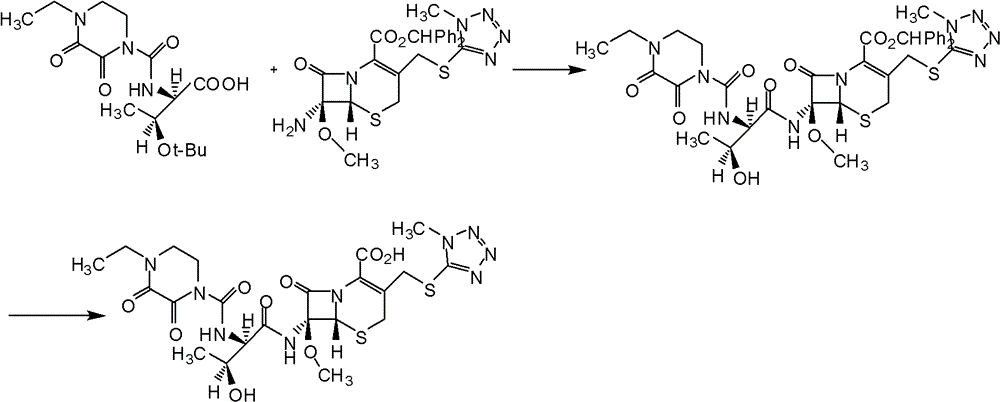
Embodiment 2
[0066] (1) Preparation of 7-MAC silyl compound
[0067]
[0068] 7-MAC 7-MAC silyl compound
[0069] Add 1.05kg of 7-MAC, 6.4kg of acetonitrile and 0.48kg of hexamethyldisilazane (HMDS) into the reaction tank, control the temperature at 15-20°C and stir for 45min, then cool down to below -5°C for use.
[0070] (2) preparation of cefbuperazone side chain acid chloride
[0071]
[0072] Cefbuperazone Side Chain Cefbuperazone Side Chain Acid Chloride
[0073] Add 0.57kg of cefbuperazone side chain, 0.19kg of tetrahydrofuran and 5.3kg of dichloromethane in the reaction tank, after stirring and dissolving, cool down to -30~-25°C, slowly add 0.466kg of phosphorus trichloride dropwise, drop During the addition process, the temperature was controlled not to exceed -20°C, and the dripping was completed within 30 minutes, and the reaction was stirred for another 30 minutes.
[0074] (3) preparation of cefbuperazone diphenylmethyl ester
[0075]
[0076] Cefbuperazone Side C...
Embodiment 3
[0083] (1) Preparation of 7-MAC silyl compound
[0084]
[0085] 7-MAC 7-MAC silyl compound
[0086] Add 1.05kg of 7-MAC, 6.4kg of N,N-dimethylformamide and 0.22kg of trimethylchlorosilane into the reaction tank, control the temperature at 25-30°C and stir for 35min, then cool down to below -5°C and wait use.
[0087] (2) preparation of cefbuperazone side chain acid chloride
[0088]
[0089] Cefbuperazone Side Chain Cefbuperazone Side Chain Acid Chloride
[0090] Add 0.61kg of cefbuperazone side chain, 0.19kg of tetrahydrofuran and 5.3kg of dichloromethane into the reaction tank. After stirring and dissolving, drop the temperature to -30~-25°C and slowly add 0.39kg of phosphorus oxychloride dropwise. During the process, the temperature was controlled not to exceed -20°C, and the dripping was completed within 30 minutes, and the reaction was stirred for another 30 minutes.
[0091] (3) preparation of cefbuperazone diphenylmethyl ester
[0092]
[0093] Cefbuperaz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com