Cefbuperazone pharmaceutical composition and preparation method thereof
A technology of cefbuperazone and cefbuperazone acid, which is applied in the field of cephalosporin antibiotic pharmaceutical composition and its preparation, can solve the problems of uneven mixing and easy layering of the cefbuperazone pharmaceutical composition, and achieve good fluidity and dissolution fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Cefrazone Acid Granules
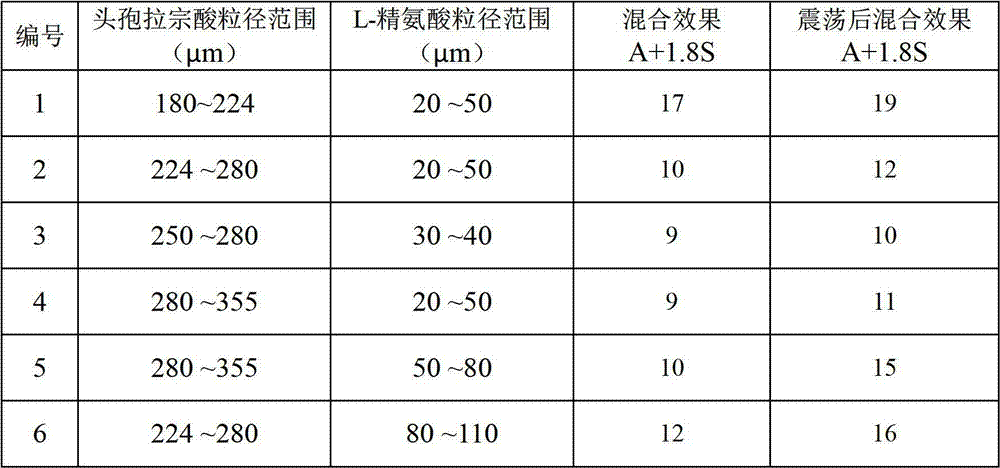
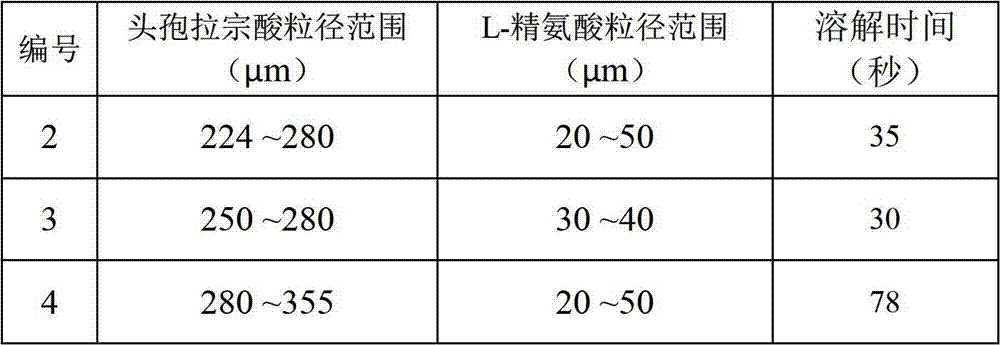
[0025] Get 4 kilograms of cefbuperazone acid crystals, after being pulverized by a pulverizer, first pass through a 60-mesh sieve, and the resulting product is placed on a 70-mesh sieve and vibrated until no particles are sieved out to obtain cefbuperazone with a particle size range of 224 to 280 μm acid granules.
Embodiment 2
[0027] Preparation of L-arginine particles
[0028] Take 1 kg of L-arginine, pulverize it in a jet mill for 15 minutes, measure the particle size, and obtain L-arginine particles with a particle size ranging from 20 to 50 μm.
Embodiment 3
[0030] In this example, the aseptic co-solvent was screened based on the water-soluble time, clarity, and gas production of the aseptic co-solvent and the cefbuperazone acid composition. The method adopted is: add 1g of the composition (0.8g of cephrazone acid + 0.2g of auxiliary agent) to 50ml of water, and shake slightly. The results are shown in Table 1.
[0031] Table 1. Dissolving situation of cefbuperazone pharmaceutical composition
[0032] Sterile Auxiliary Clarity Gas production Sodium carbonate clarify gas production sodium bicarbonate clarify gas production L-Arginine clarify no gas
[0033] It can be seen from the data in the above table that sodium carbonate and sodium bicarbonate have the disadvantage of producing a large amount of gas, which will cause an increase in the internal pressure of the bottle when used, so L-arginine that does not produce gas is selected as an auxiliary agent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com