Drug composition of cefoperazone sodium and tazobactam sodium and preparation method thereof
A technology of cefoperazone sodium and tazobactam sodium, which is applied in the field of medicine, can solve problems such as the increase of polymer impurity content, the decrease of drug active ingredient content, and the impact on product stability, effectiveness, and safety, achieving good stability, The effect of reducing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
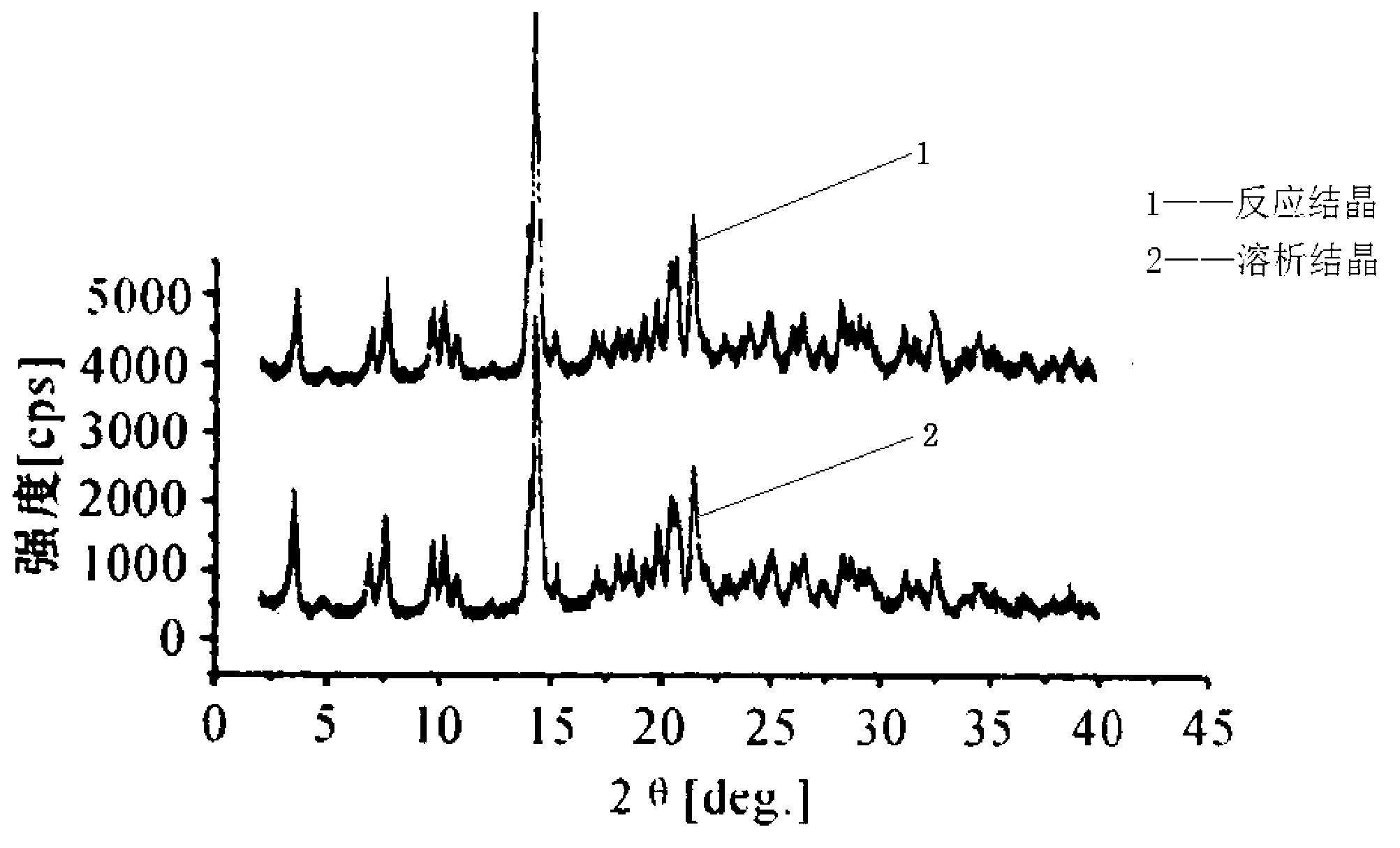
Image
Examples
Embodiment 1
[0043] [Example 1] Preparation of Cefoperazone Sodium Compound
[0044] 1) Dissolve 12kg of crude cefoperazone sodium in 10L of water to obtain a 1.2kg / L aqueous solution of cefoperazone sodium;
[0045] 2) Add activated carbon to the cefoperazone sodium aqueous solution in step 1) (the amount of activated carbon is 0.3% g / ml of the volume of the cefoperazone sodium aqueous solution), stir, suction filter, and take the filtrate;
[0046]3) Add 50L of organic solvent A to the filtrate of step 2) at a stirring speed of 50r / min (the flow rate of organic solvent A is 13L / min) to form a turbid solution, wherein the organic solvent A is methanol A mixed solvent composed of tetrahydrofuran and tetrahydrofuran at a volume ratio of 2:1;
[0047] 4) After adjusting the stirring speed to 15r / min, add 300L of organic solvent B to the turbid solution in step 3) (the flow rate of organic solvent B is 12L / min). It is a mixed solvent composed of isobutyl methyl ketone and dimethyl formamide...
Embodiment 2
[0050] [embodiment 2] the preparation of cefoperazone sodium compound
[0051] 1) Dissolve 26kg of crude cefoperazone sodium in 10L of water to obtain a 2.6kg / L aqueous solution of cefoperazone sodium;
[0052] 2) Add activated carbon to the cefoperazone sodium aqueous solution in step 1) (the amount of activated carbon is 0.3% g / ml of the volume of the cefoperazone sodium aqueous solution), stir, suction filter, and take the filtrate;
[0053] 3) Add 100L of organic solvent A to the filtrate of step 2) at a stirring speed of 60r / min (the flow rate of organic solvent A is 20L / min) to form a turbid solution, wherein the organic solvent A is methanol A mixed solvent composed of tetrahydrofuran and tetrahydrofuran at a volume ratio of 5:1;
[0054] 4) After adjusting the stirring speed to 25r / min, add 200L of organic solvent B to the turbid solution in step 3) (the flow rate of organic solvent B is 6L / min). It is a mixed solvent composed of isobutyl methyl ketone and dimethyl f...
Embodiment 3
[0057] [embodiment 3] the preparation of cefoperazone sodium compound
[0058] 1) Dissolve 20kg of crude cefoperazone sodium in 10L of water to obtain a 2.0kg / L aqueous solution of cefoperazone sodium;
[0059] 2) Add activated carbon to the cefoperazone sodium aqueous solution in step 1) (the amount of activated carbon is 0.3% g / ml of the volume of the cefoperazone sodium aqueous solution), stir, suction filter, and take the filtrate;
[0060] 3) Add 80L of organic solvent A to the filtrate of step 2) at a stirring speed of 55r / min (the flow rate of organic solvent A is 15L / min) to form a turbid solution, wherein the organic solvent A is methanol A mixed solvent composed of tetrahydrofuran and tetrahydrofuran at a volume ratio of 3.5:1;
[0061] 4) After adjusting the stirring speed to 20r / min, add 100L of organic solvent B to the turbid solution in step 3) (the flow rate of organic solvent B is 8L / min). It is a mixed solvent composed of isobutyl methyl ketone and dimethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com