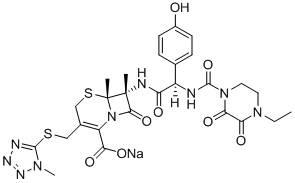
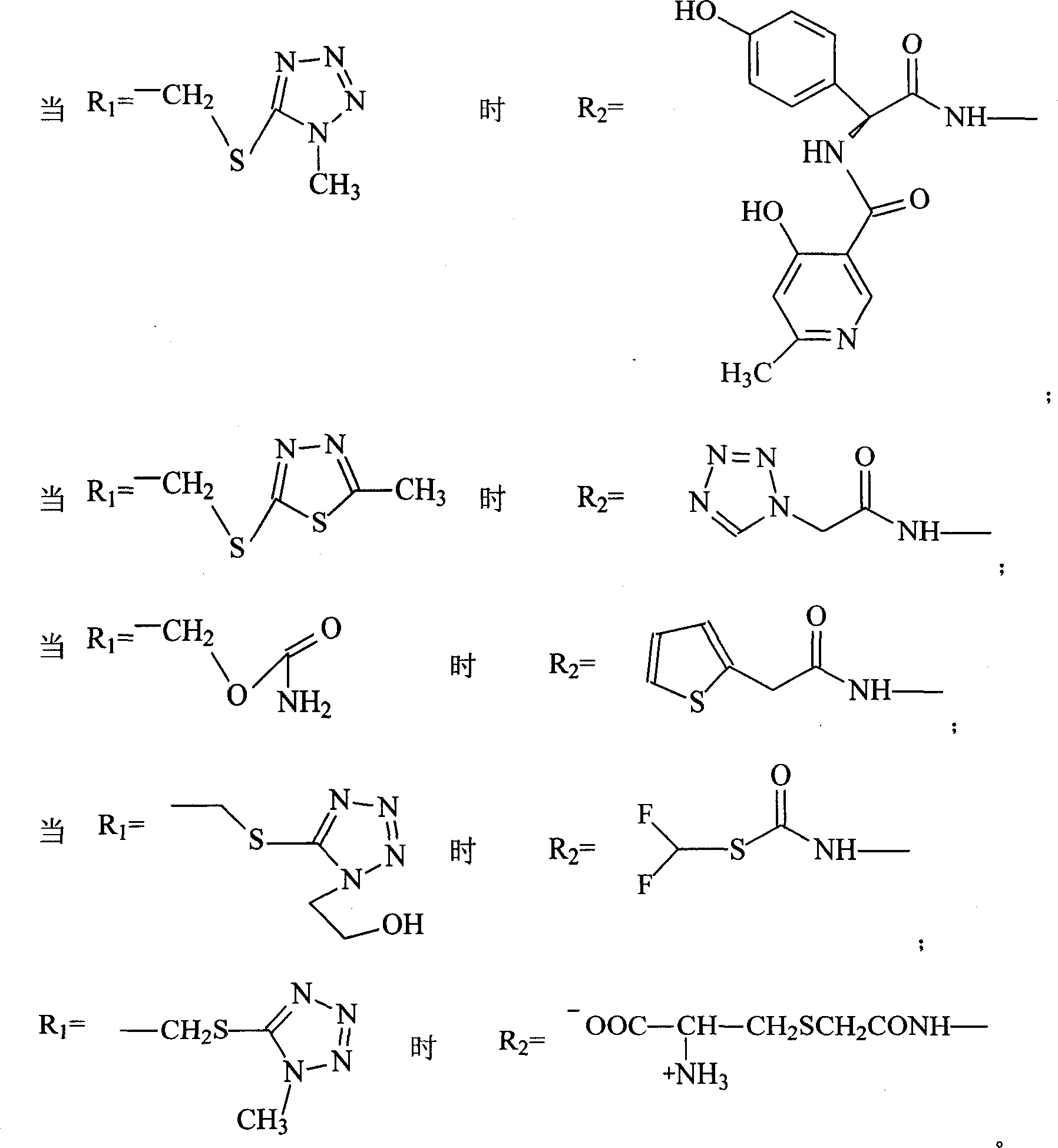
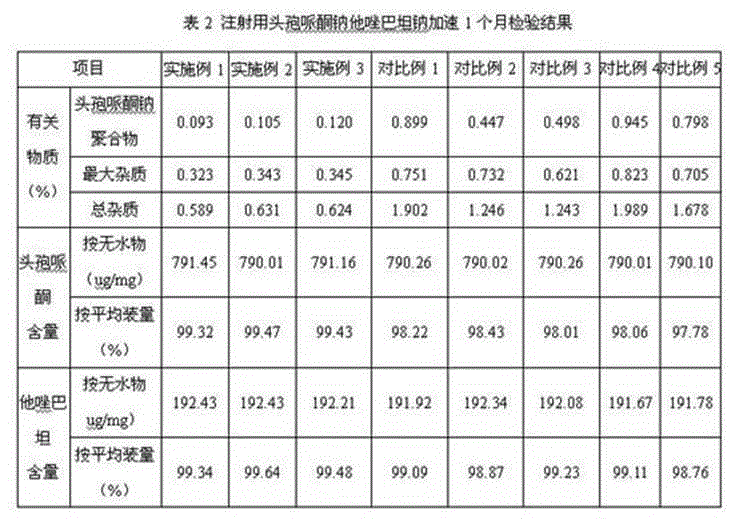
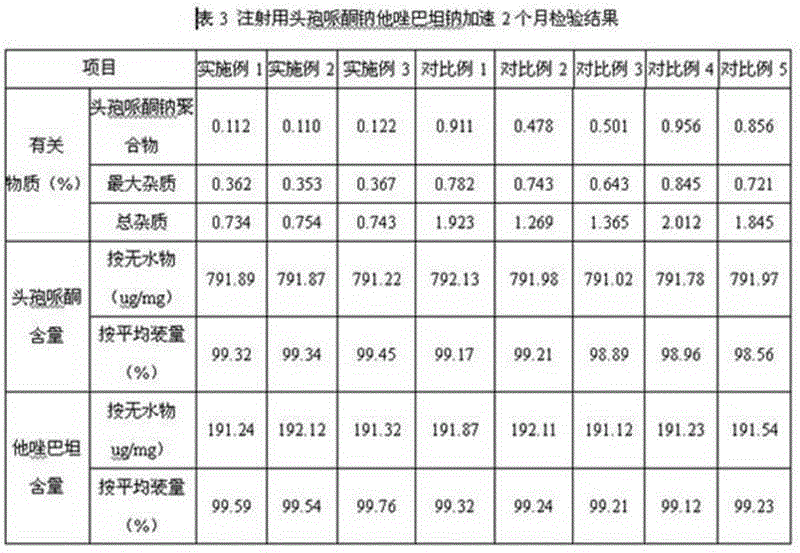
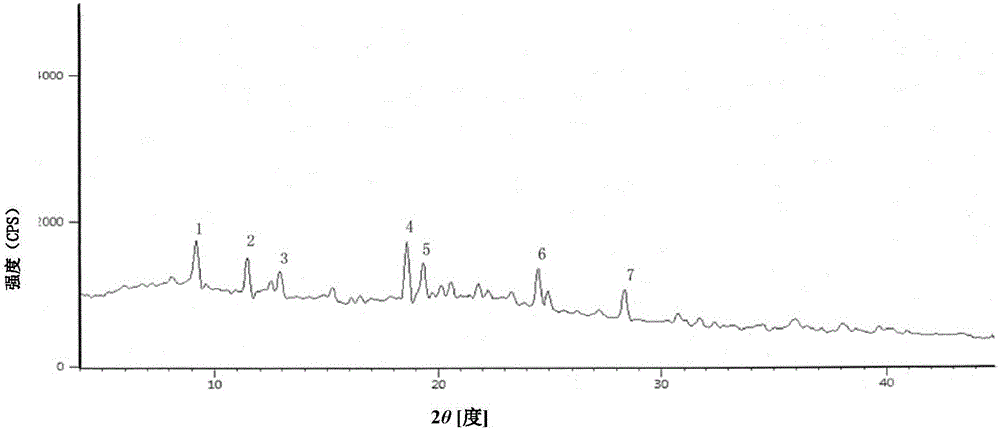
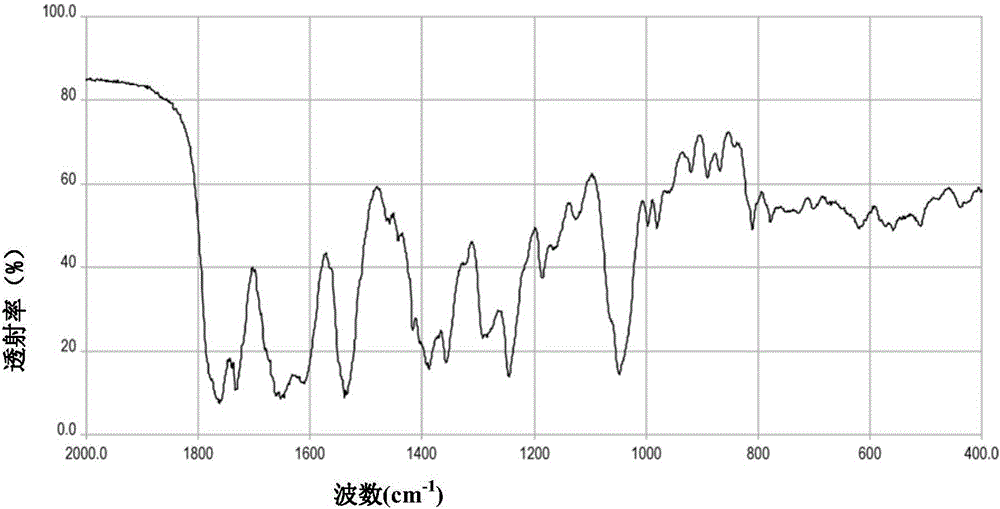
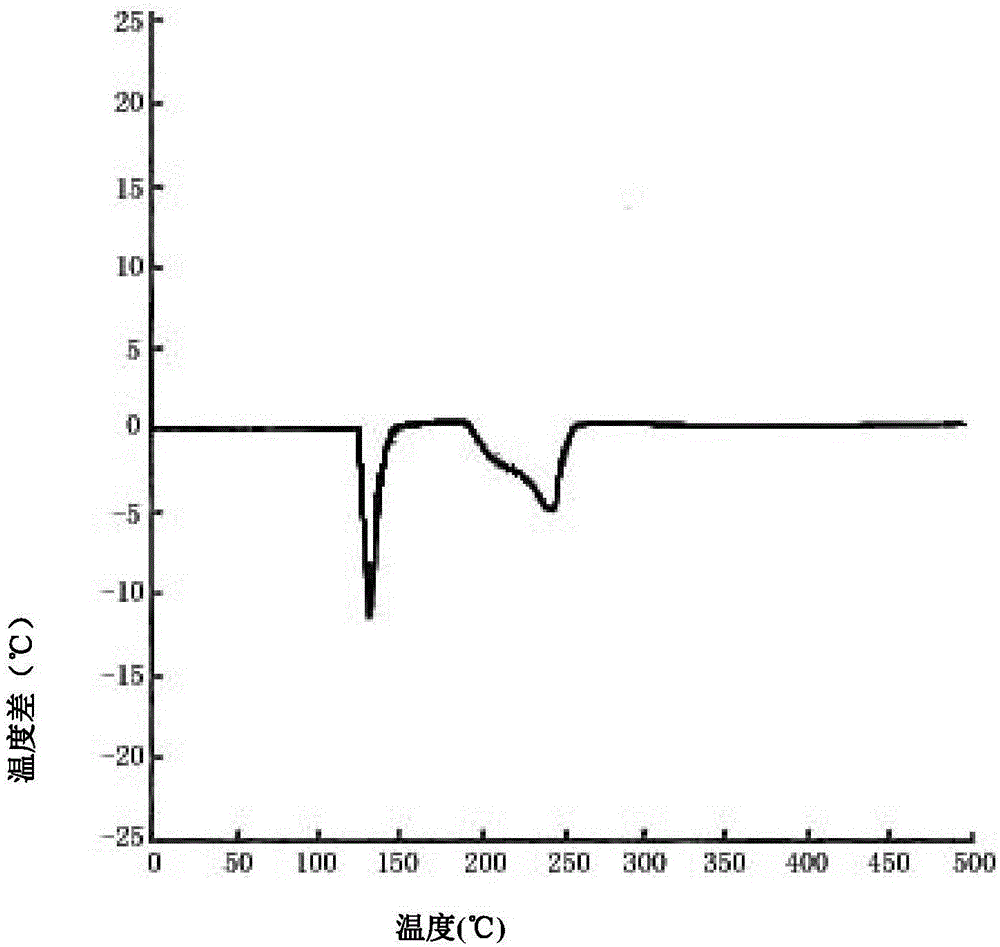
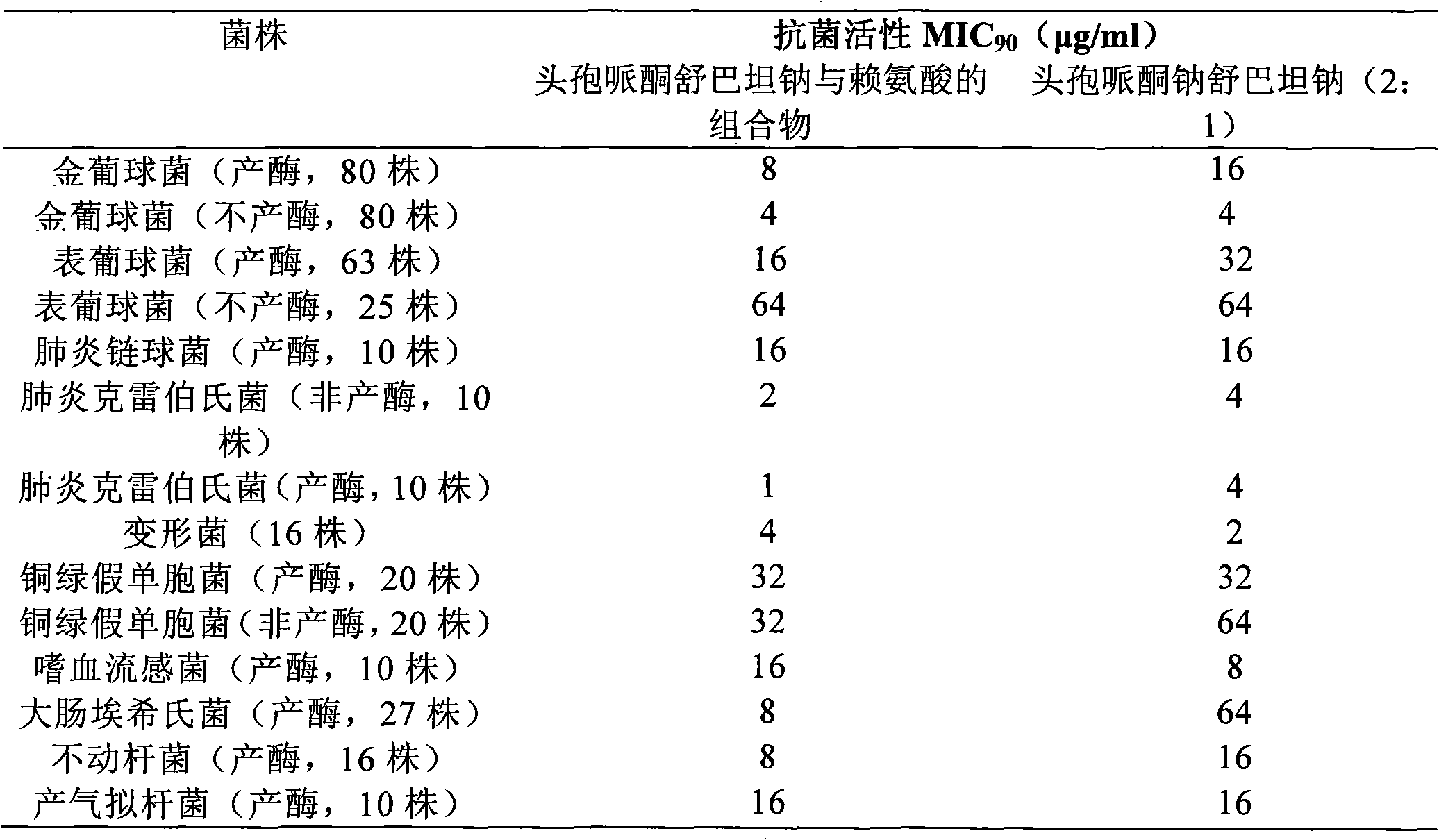
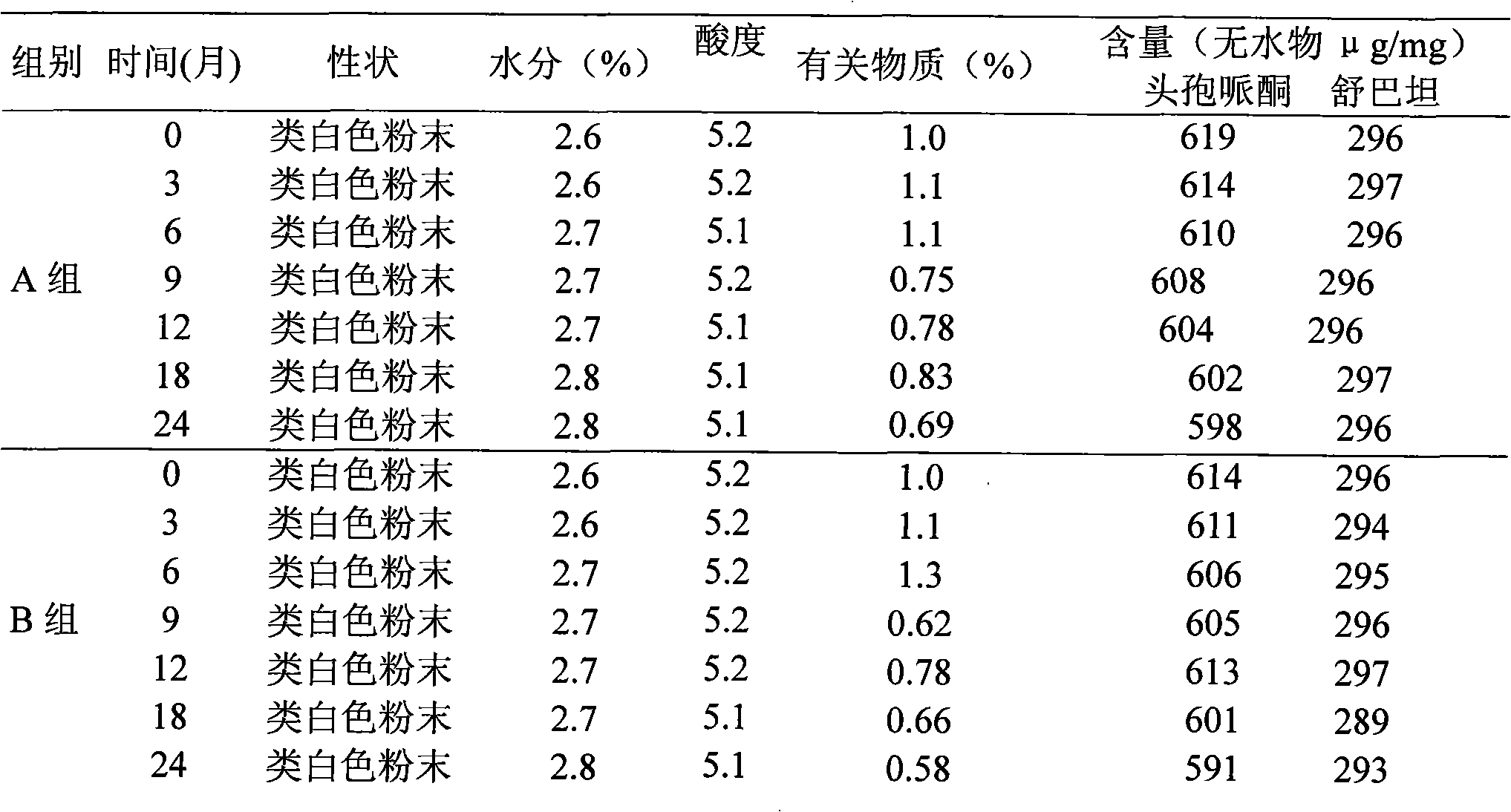
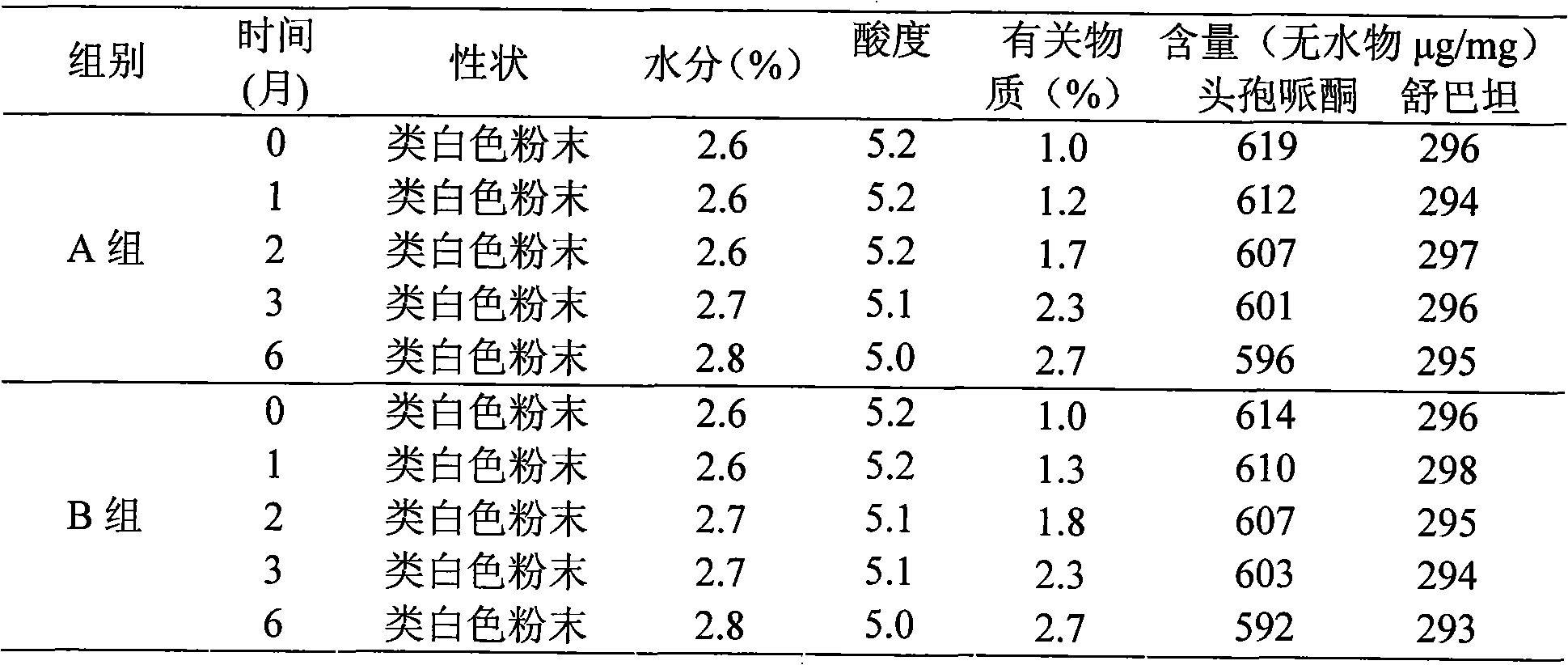
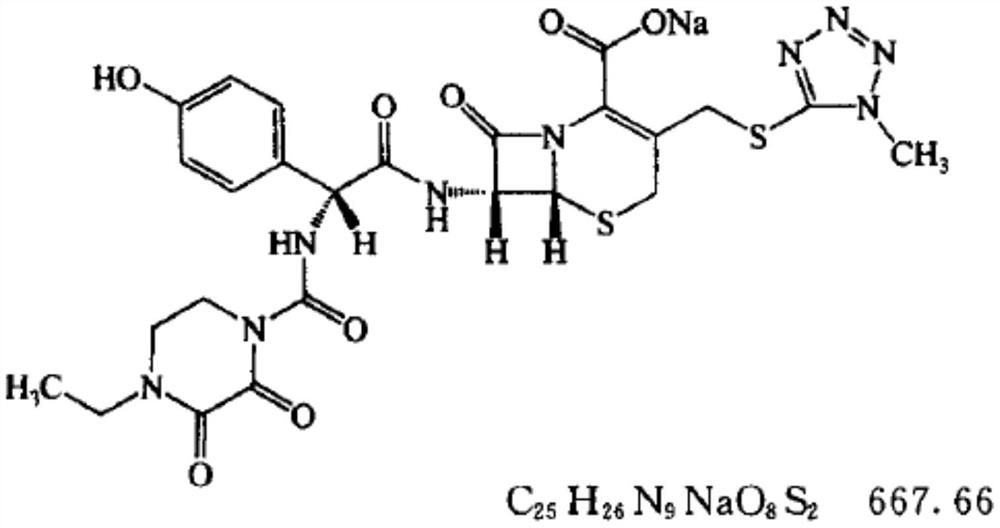
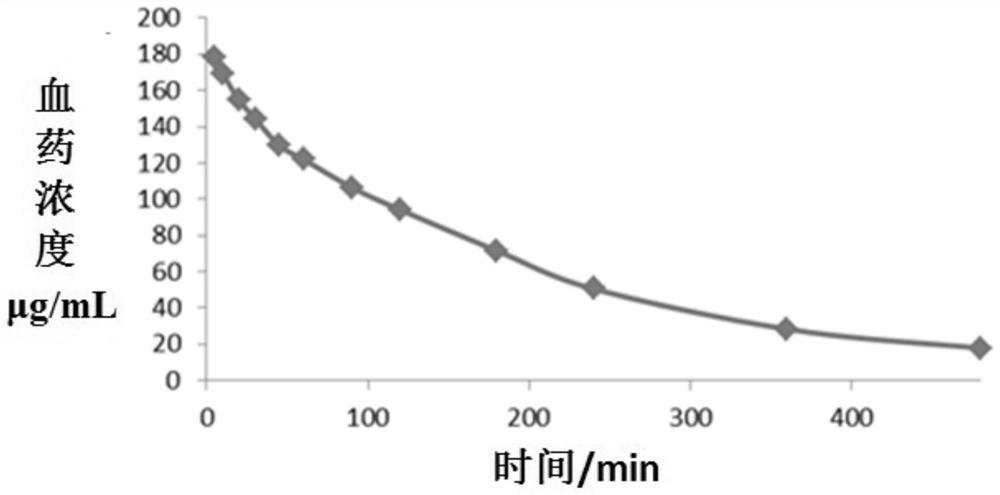
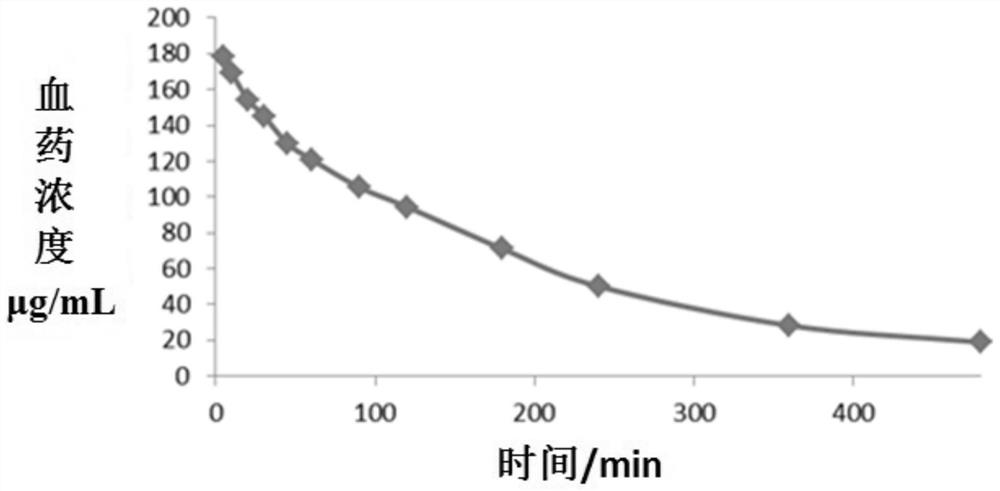
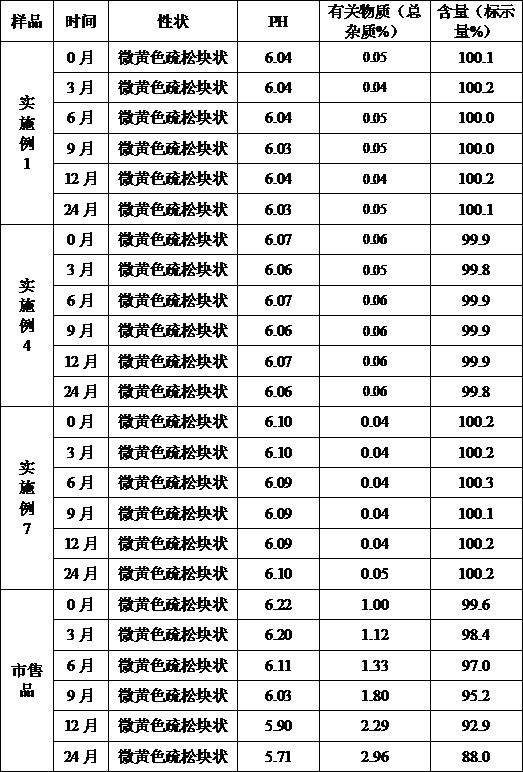
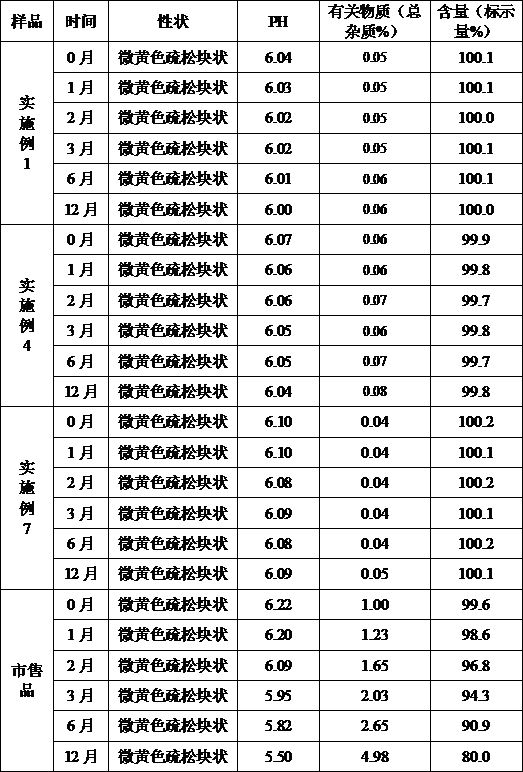
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
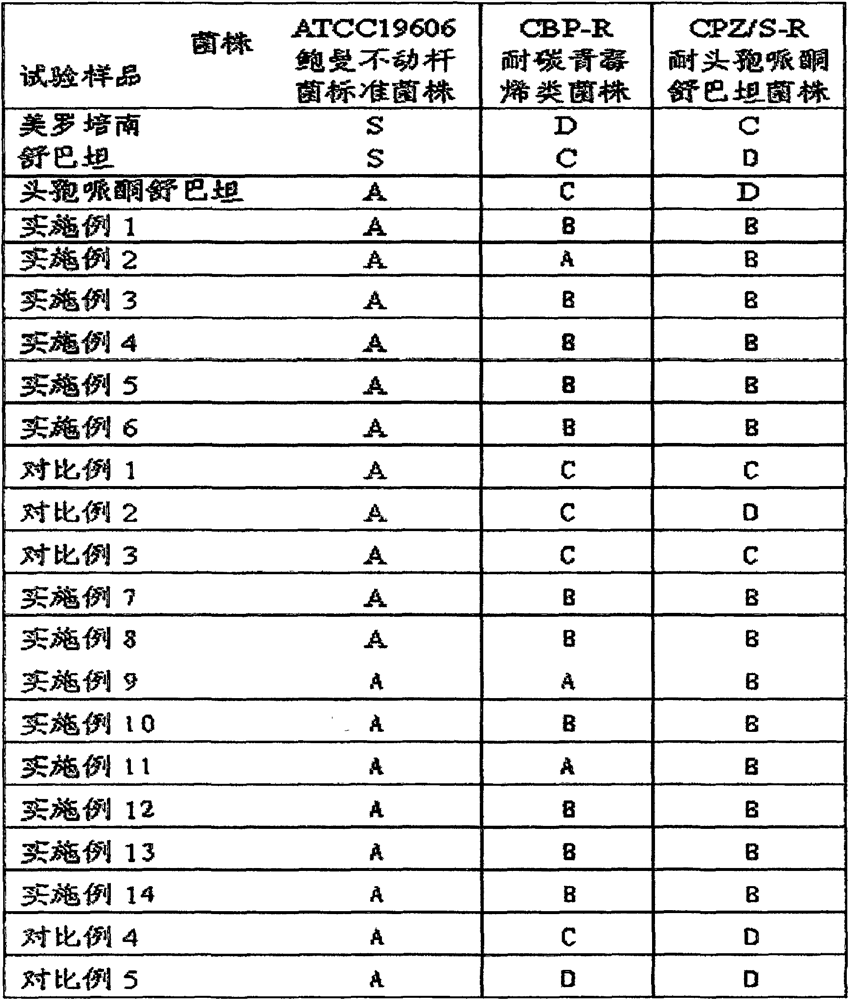
51 results about "Cefoperazon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
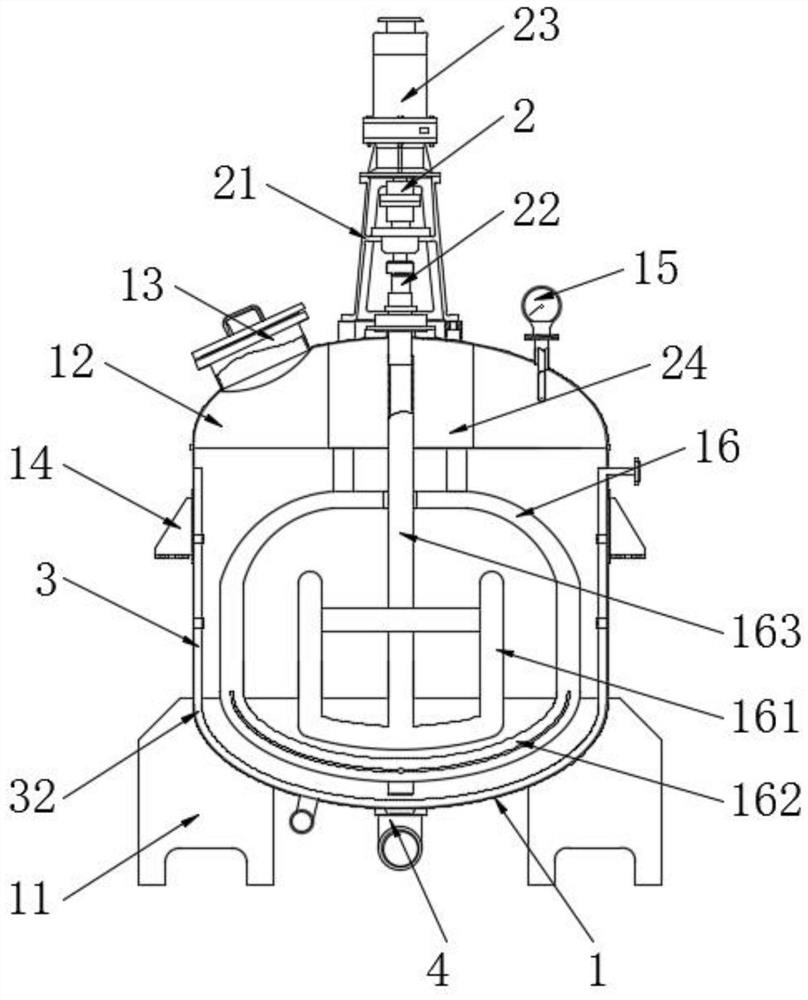
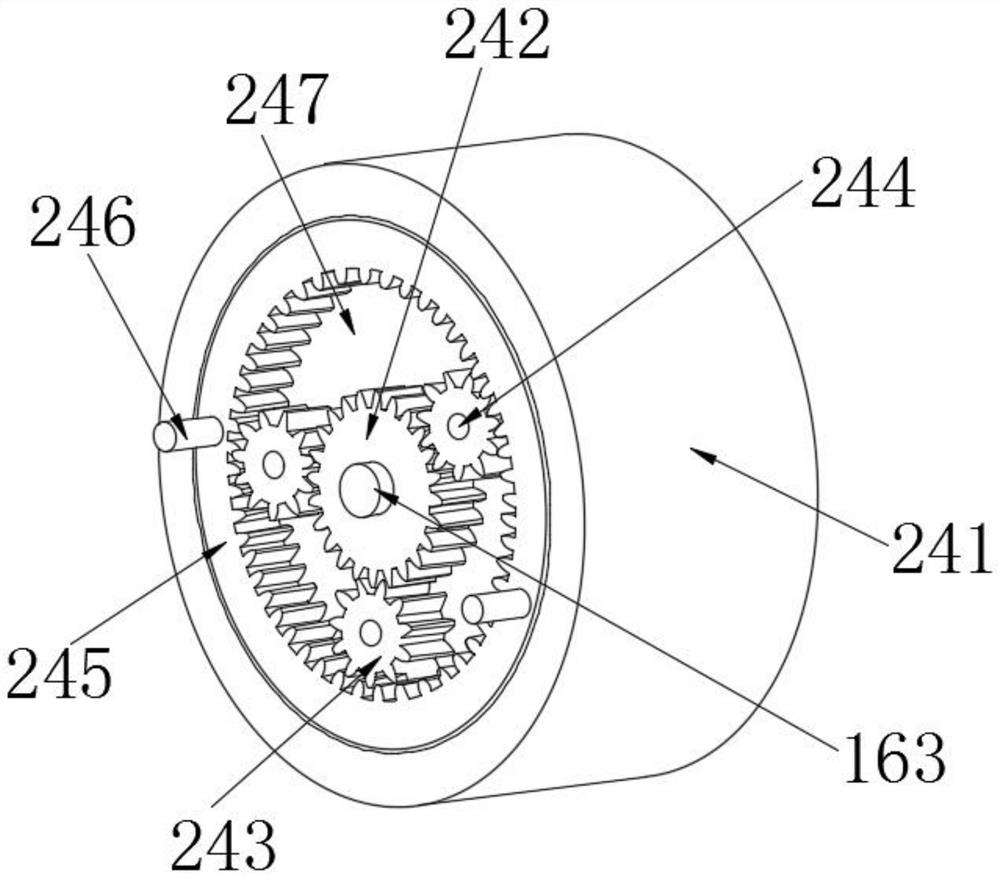
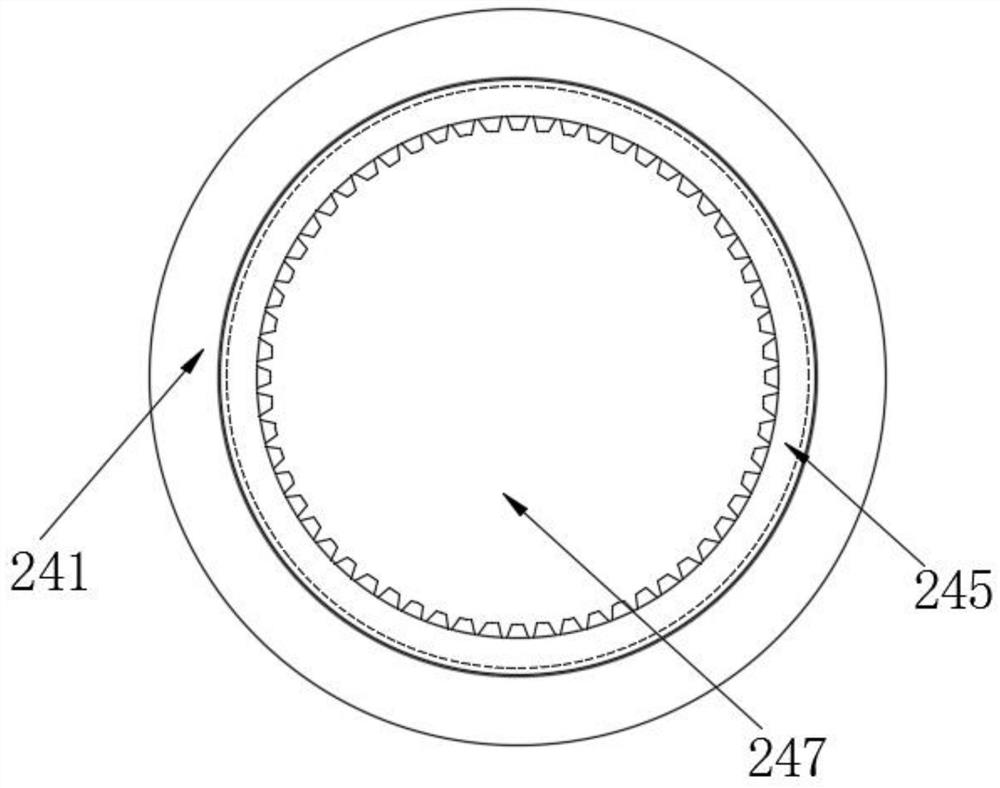
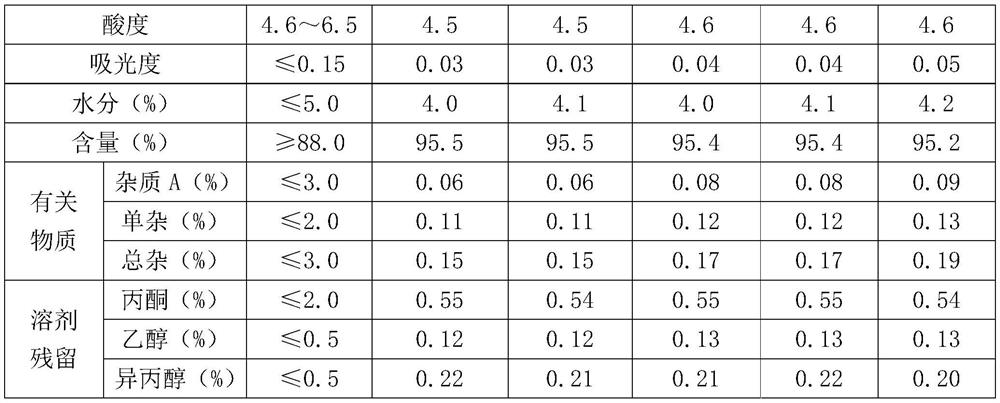
Method for crystallizing cefoperazone sodium
The invention aims to provide a method for crystallizing cefoperazone sodium, which comprises the following steps of: dissolving cefobid in a solvent, and adding a salt forming agent to adjust the pH to between 6.2 and 6.8 until the solid is completely dissolved; and filtering, adding the filtrate into the solvent under the ultrasonic condition to separate out crystals, filtering, washing and leaching the crystals, and performing vacuum drying to obtain the cefoperazone sodium. Aiming at the problems in the industrial crystallization of the cefoperazone sodium, the ultrasonic radiation has strong orienting effect, supplements and enhances the wave action required by the formation of critical crystal nucleus, can accelerate graining process and promote the quick generation of the crystal nucleus, and achieves the effect of quickly graining without seeding by seed crystals; meanwhile, the ultrasonic in the liquid medium can make mass points in the medium obtain high acceleration, has the effect of cavitation, and can prevent the accumulation during crystal growth to make the size distribution of the crystals uniform.
Owner:FUJIAN FUKANG PHARMA
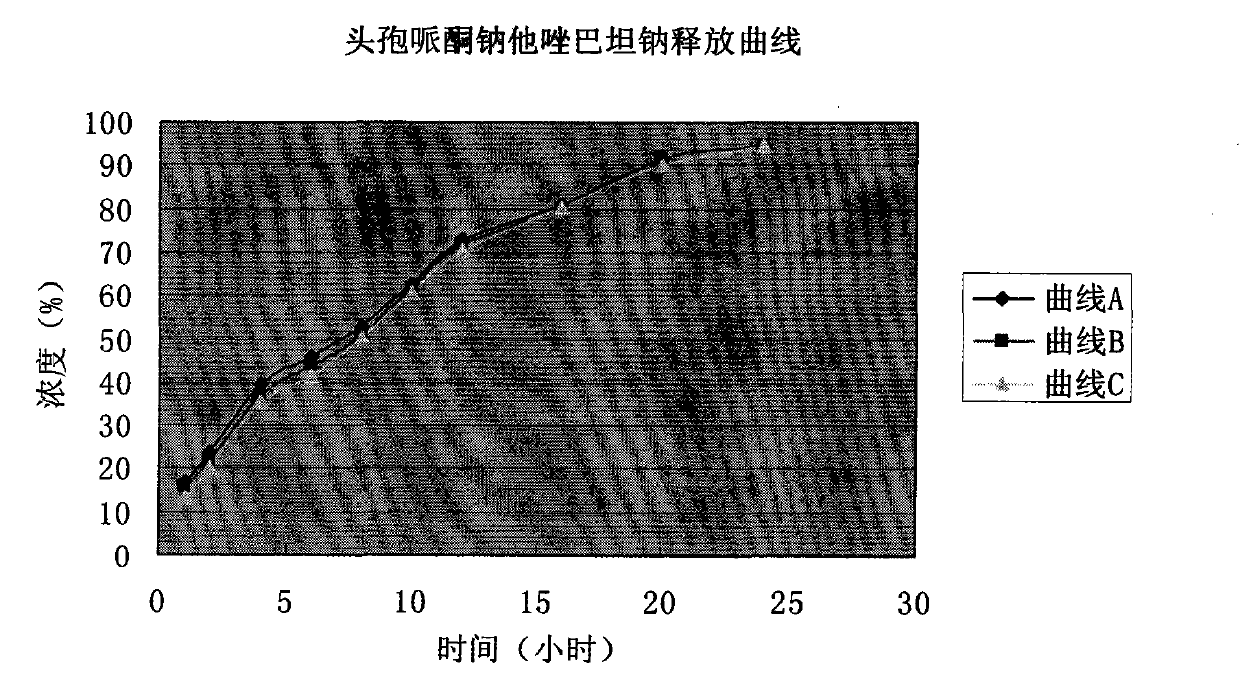
Suspension powder injection of cefoperazone sodium and tazobactam sodium pharmaceutical composition and new application thereof
InactiveCN101632677AUnexpected effectImprove stabilityPowder deliveryUrinary disorderFreeze-dryingBULK ACTIVE INGREDIENT
The invention discloses a suspension powder injection with a cefoperazone sodium and tazobactam sodium pharmaceutical composition as an active ingredient, and comprises the following components: 4 parts of the cefoperazone sodium, 1 part of the tazobactam sodium, 5-30 parts of an emulsifier, 1-15 parts of an auxiliary emulsifier and 1-40 parts of a freeze-drying support agent. The invention further discloses an application thereof in preparing medicines for treating cystitis.
Owner:HAINAN YONGTIAN PHARMA INST
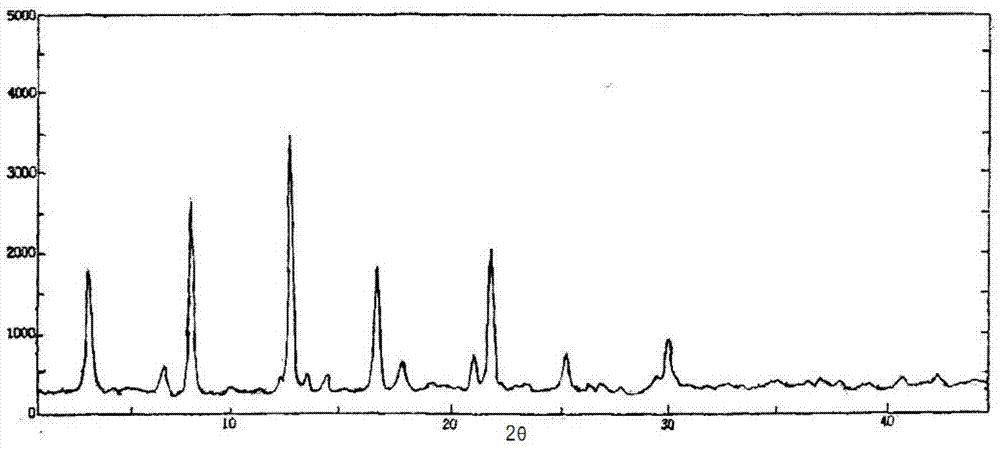
Cefoperazone sodium compound prepared by using fluid mechanics principle and preparation comprising cefoperazone sodium compound
InactiveCN106432273AEasy to manufactureSmall particle sizeOrganic active ingredientsOrganic chemistryMedical productX-ray
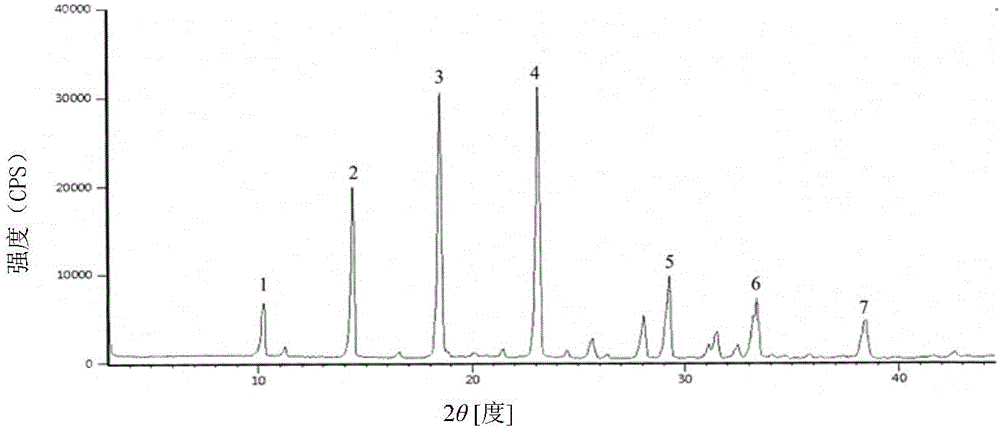
The invention discloses a cefoperazone sodium compound prepared by using the fluid mechanics principle. Research&Development and Industrialization Project of High-end Medical Product Refinement Crystallization Technologies wins the second prize of national scientific and technological progress in 2015, and the fluid mechanics principle crystallization technology belongs to one of the high-end medical product refinement crystallization technologies. The cefoperazone sodium compound is determined by using X-ray powder diffraction, and the main characteristic peaks represented by the diffraction angle 2 theta in a map are located at 10.25+ / -0.2 degrees, 14.40+ / -0.2 degrees, 18.51+ / -0.2 degrees, 23.14+ / -0.2 degrees, 29.10+ / -0.2 degrees, 33.25+ / -0.2 degrees and 38.45+ / -0.2 degrees. Cefoperazone acid reacts with a salt forming agent, and the cefoperazone sodium compound is prepared through secondary crystallization. The operation is simple, reactants are easy to obtain, the reaction condition is mild, and the yield is high. The compound is high in purity, low in impurity content, good in fluidity and good in stability. Meanwhile, the invention further discloses a preparation prepared from cefoperazone sodium, namely, cefoperazone sodium for injection. The preparation process of the preparation is simple, no excipient is needed, and the preparation has better stability and few side effects.
Owner:陕西顿斯制药有限公司
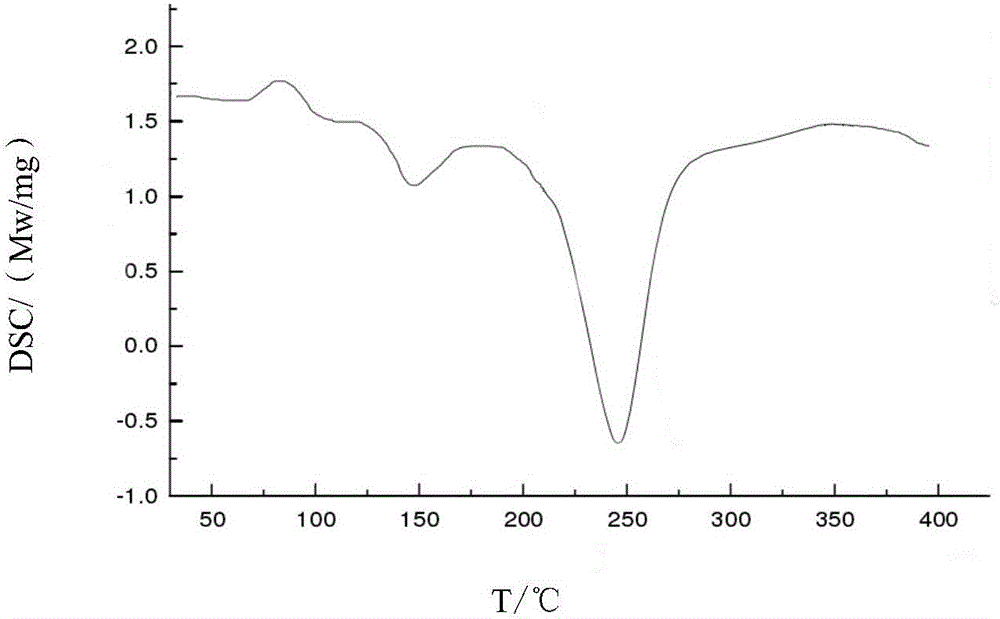
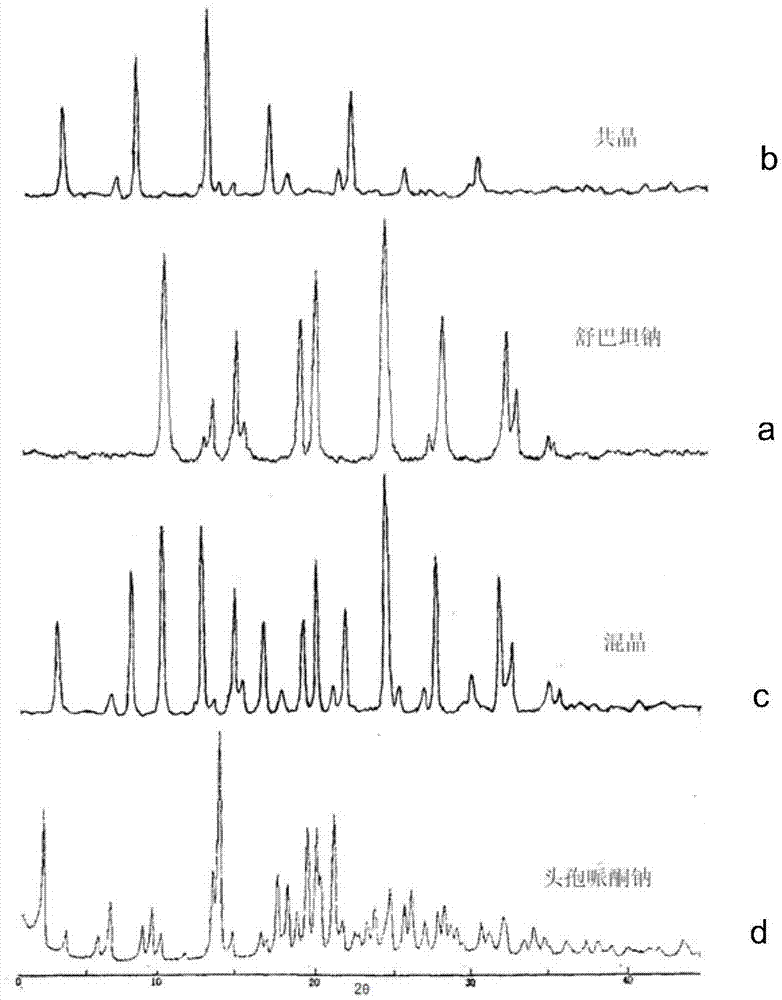
Cefoperazone sodium-sulbactam sodium eutectic crystal and composition, and preparation methods thereof
ActiveCN104844624AImprove stabilityEasy to operateAntibacterial agentsPowder deliveryPhosphateCrystallinity
The invention relates to a cefoperazone sodium-sulbactam sodium eutectic crystal and a pharmaceutical composition containing the eutectic crystal, and preparation methods thereof. The cefoperazone sodium-sulbactam sodium eutectic crystal has the advantages of concentrated distribution of crystal form grain size, good product fluidity, gloss surface, high degree of crystallinity and good stability; the preparation method of the crystal form is simple in process, easy to operate, and suitable for popularization and application in a wide range. In the compound cefoperazone sodium-sulbactam sodium preparation, through introduction of the cefoperazone sodium-sulbactam sodium eutectic crystal and a phosphate buffer to adjust the pH value, the problems that a mixed powder in the preparation is poor in uniformity, liquidity and raw material stability are solved, generation of carbon dioxide gas due to addition of a stabilizer carbonate is avoided, an obtained powder injection is diluted through conventional transfusion, then dissolved rapidly and does not produce crystallization and degradation products, the solution clarity is in accordance with provisions, the solution pH value has no obvious change, and the contents of cefoperazone sodium and sulbactam sodium are stable.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Stable cefoperazone medicine prparation
InactiveCN101036657AImprove product qualitySignificant changeAntibacterial agentsOrganic active ingredientsSodium bicarbonateSolvent
The invention discloses a stable compound preparation of cefoperazone drug, which is comprised by cefoperazone acid and latent solvent, which weight ratio is 1:0.62~0.06. The latent solvent is preferred selected from sodium carbonate and sodium bicarbonate. Related substances of the compound preparation in the invention and labelled content do not change much in influencing factor and accelerated test in 40 DEG C., which accord with the standard of pharmacopoeia, and the product quality is more stable than cefoperazone sodium during period of validity.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
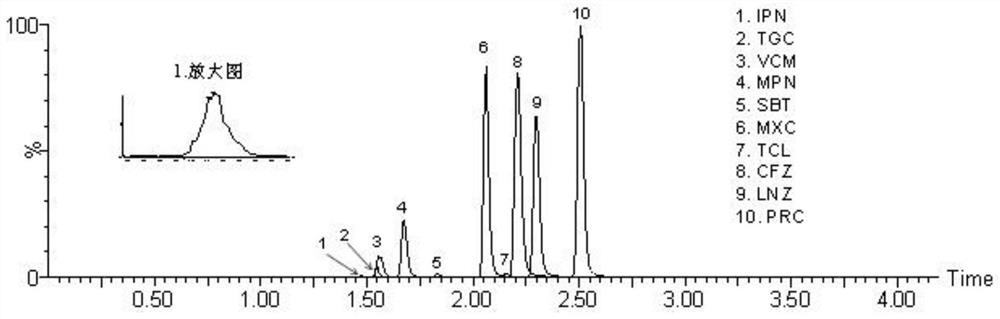
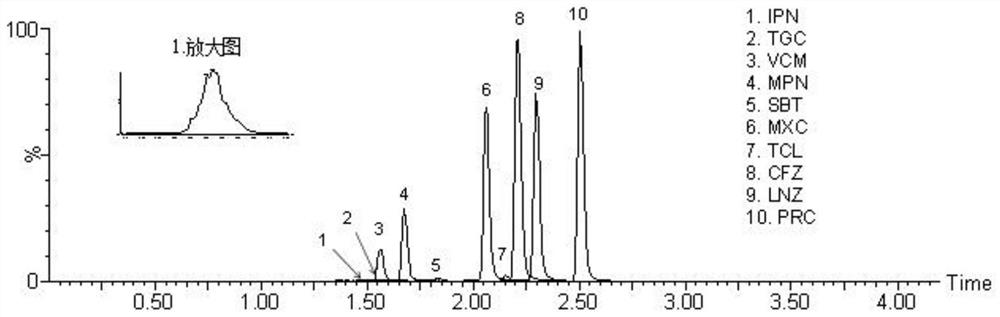
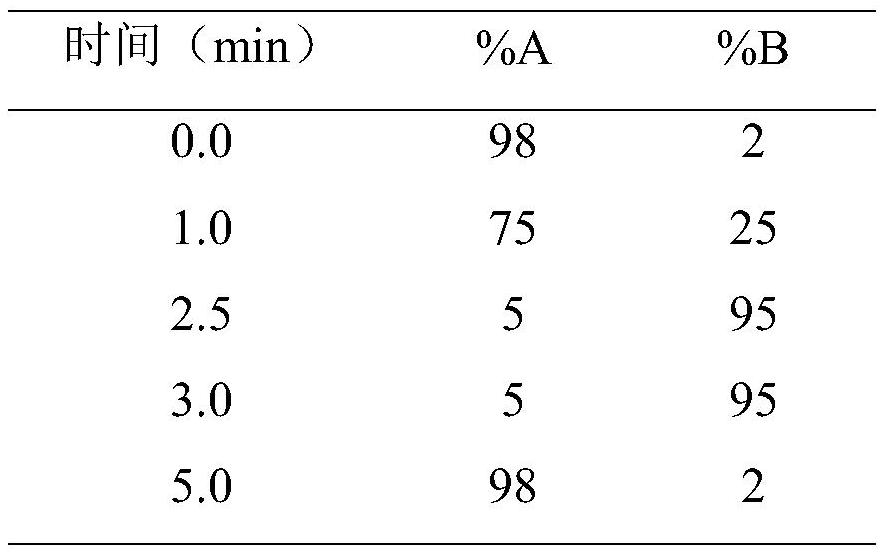
Method for detecting antibacterial agent in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention discloses a method for detecting an antibacterial agent in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antibacterial agent containssulbactam (SBT), imipenem (IPN), linezolid (LNZ), melopenem (MPN), moxifloxacin (MXC), piperacillin (PRC), tigecycline (TGC), cefoperazone (CFZ), vancomycin (VCM) and teicoplanin (TCL). The method comprises the steps: detecting the content of the antibacterial drug in the pretreated serum by adopting an ultra-high performance liquid chromatography tandem mass spectrometry method, quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of a standard substance to an internal standard substance as an X axisand the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of a target drug in the serum. According to the method, the pretreatment process is simple, the sensitivity is high, the specificity is high, separation and detection of the antibacterial agent are completed within 5 min, and a reliable detection method is provided for monitoring the treatment concentration of the antibacterial agent clinically.
Owner:南京品生医学检验实验室有限公司
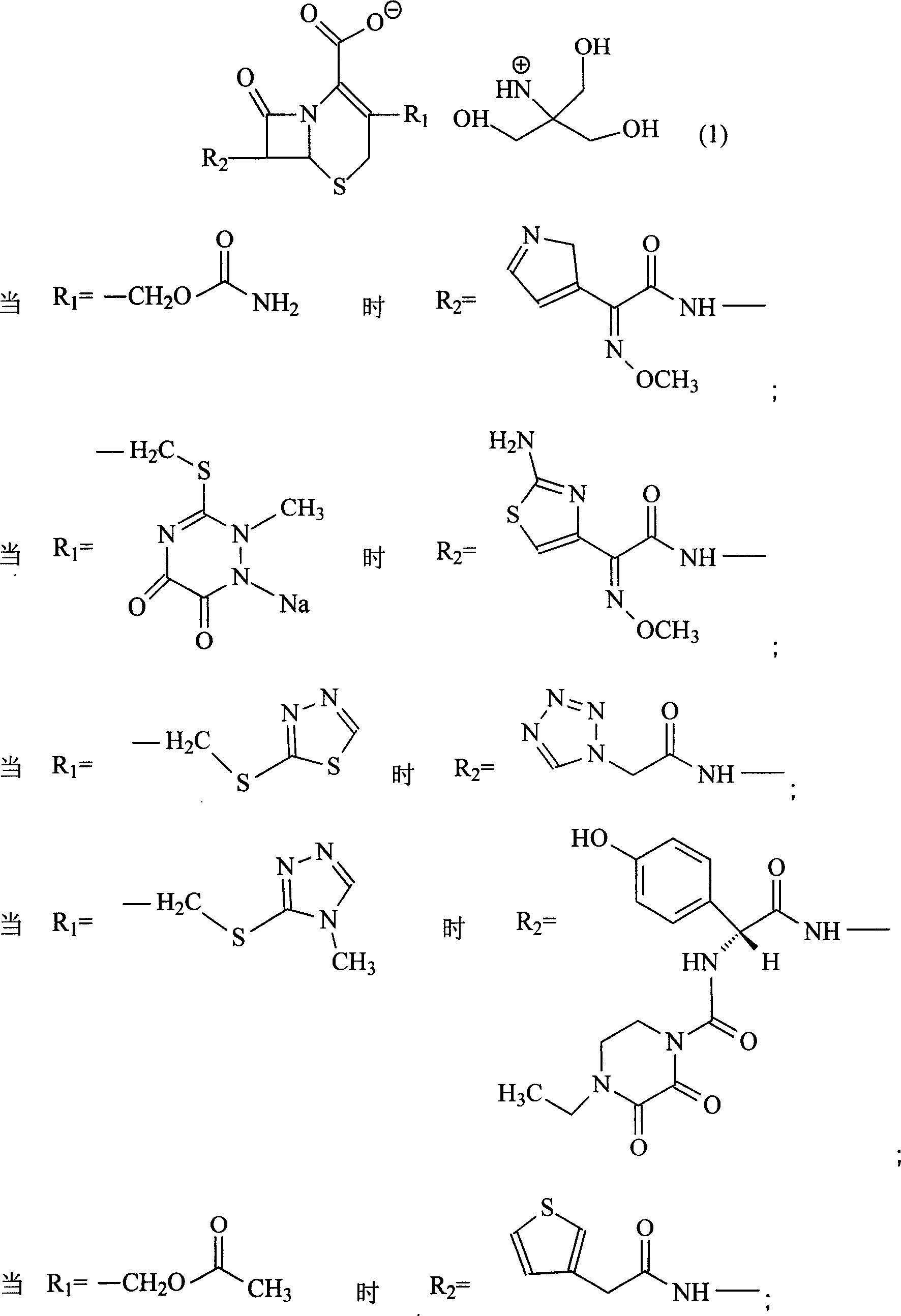
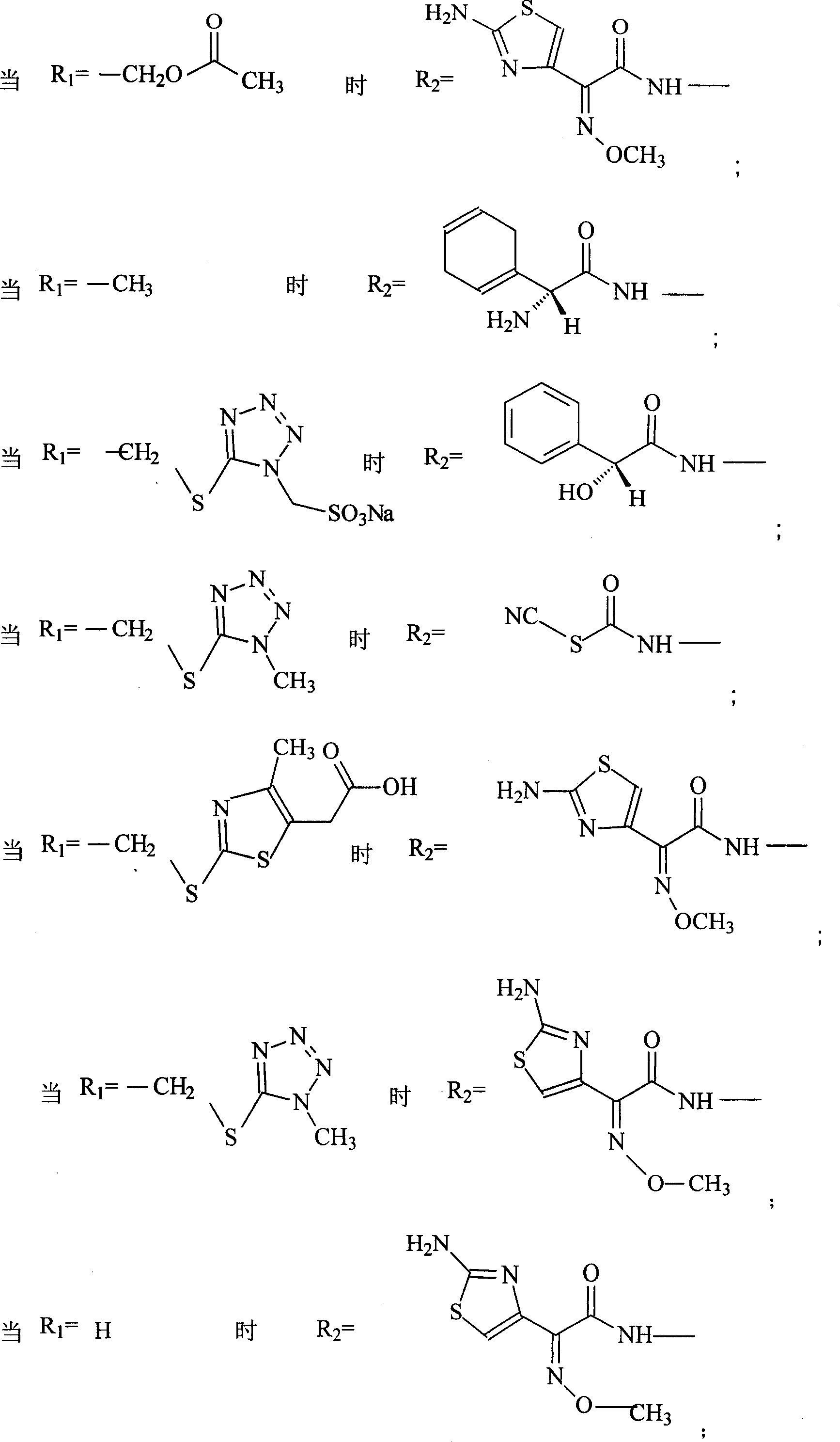
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
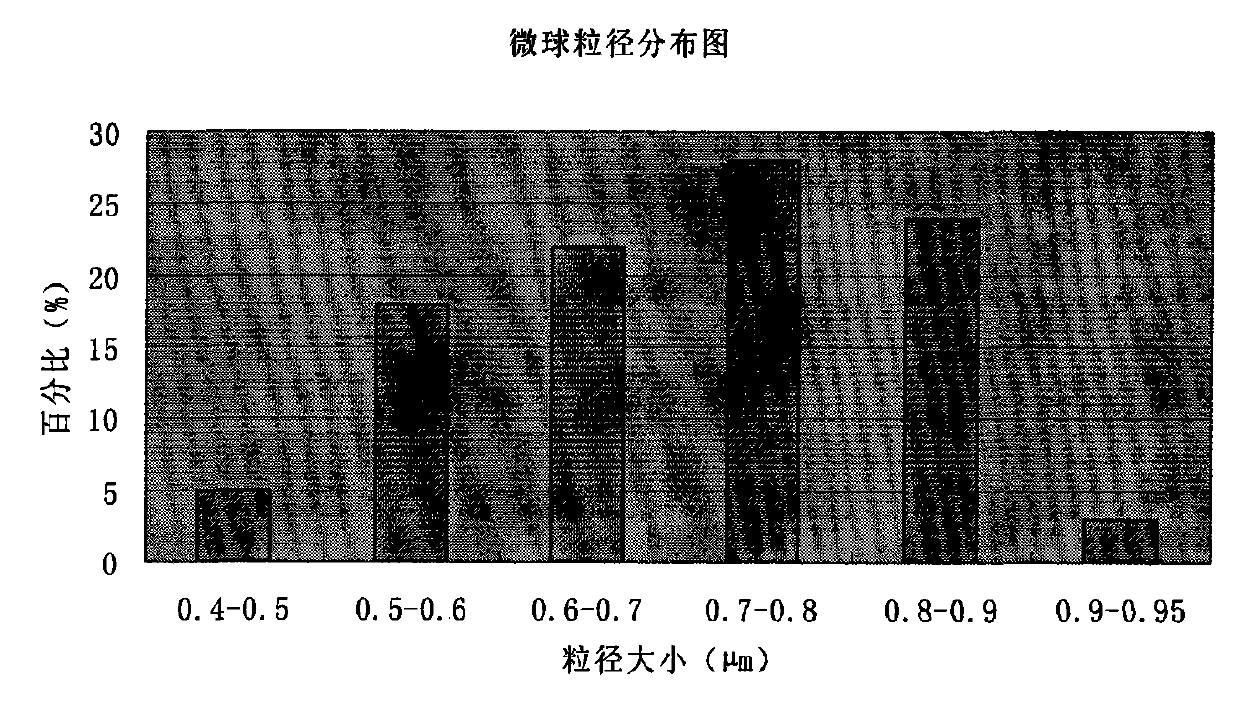
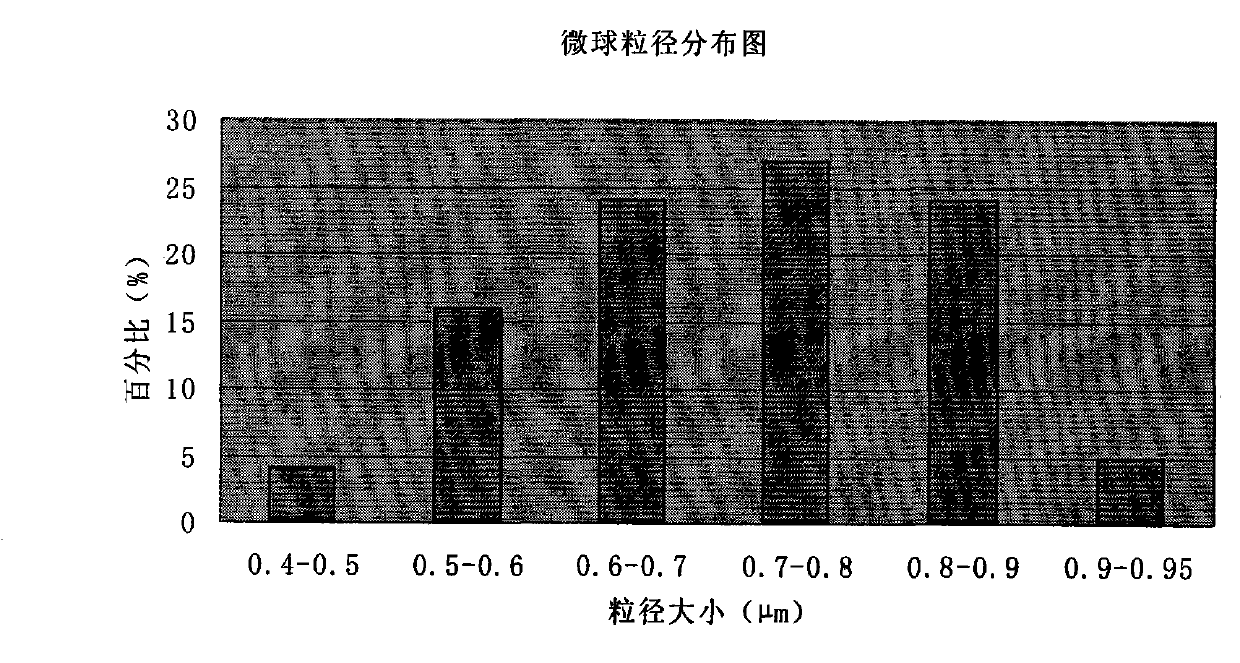
Cefoperazone sodium and tazobactam sodium medicinal composition microsphere injection
InactiveCN101912403AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsMicrosphereGlycerol
The invention discloses cefoperazone sodium and tazobactam sodium medicinal composition microsphere injection, which is characterized by comprising 4 to 8 weight parts of cefoperazone sodium, 1 to 2 weight parts of tazobactam sodium, 2.5 to 5 weight parts of PLA, 1.25 to 2.5 weight parts of Span60, 1 to 2 weight parts of glycerol monostearate and 1 to 2 weight parts of glucose. Compared with the prior art, the cefoperazone sodium and tazobactam sodium medicinal composition microsphere injection of the invention has the advantages of high stability, high entrapment rate, high-repeatability preparation process suitable for industrial production, uniform particle distribution, little solvent residue, high injectability and good sustained-release property.
Owner:HAINAN LINGKANG PHARMA CO LTD
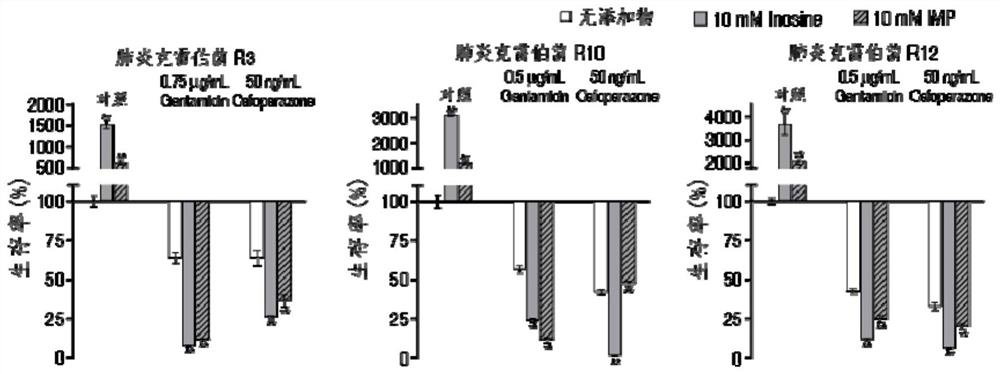
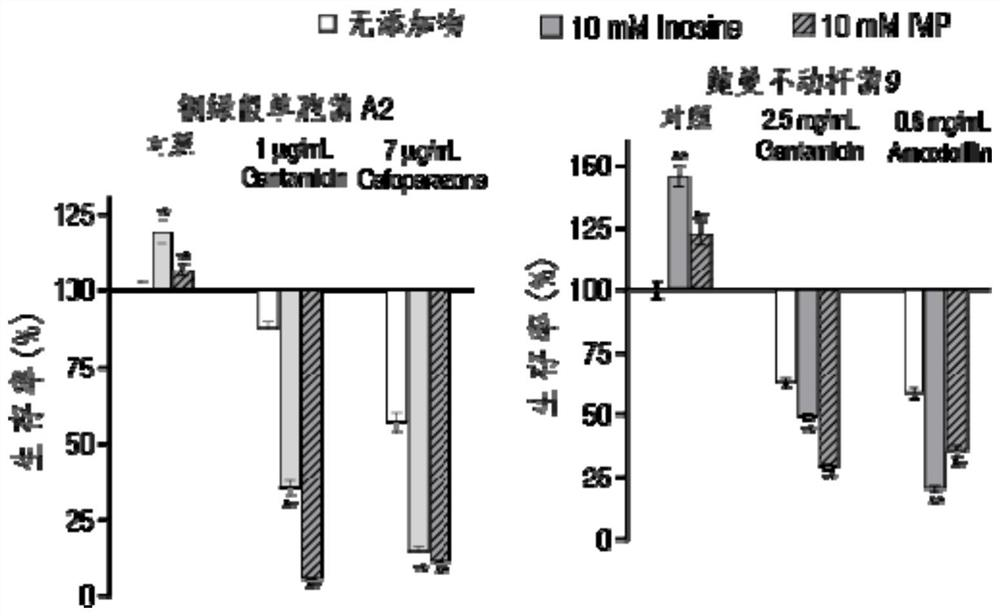
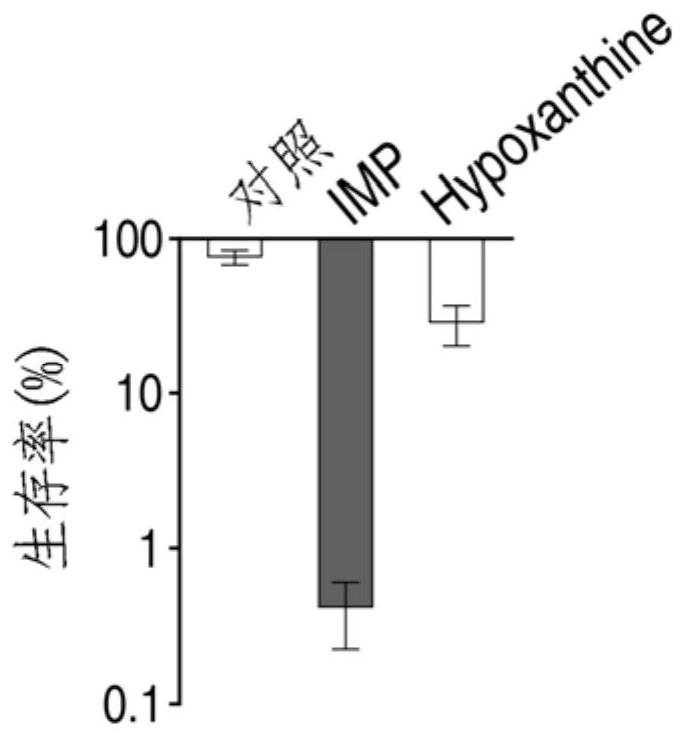
Application of hypoxanthine nucleotide in preparation of anti-infective drugs
PendingCN112569251AGood effectDelay drug resistanceAntibacterial agentsOrganic active ingredientsMulti resistant bacteriaK pneumoniae
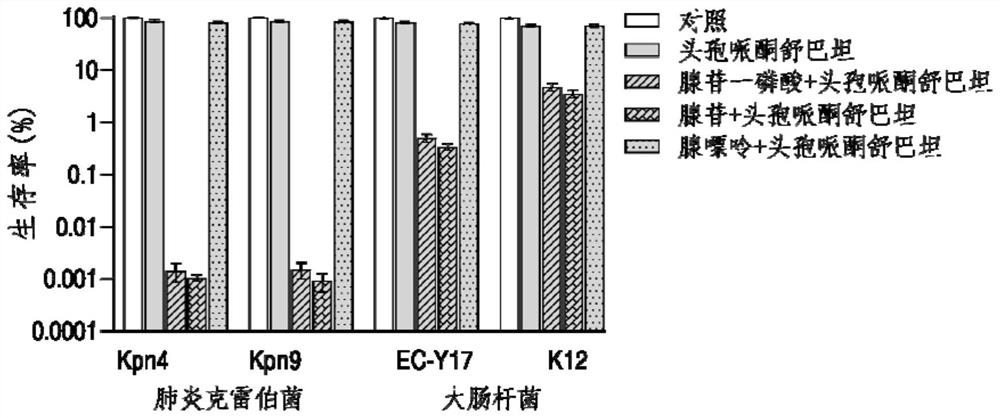
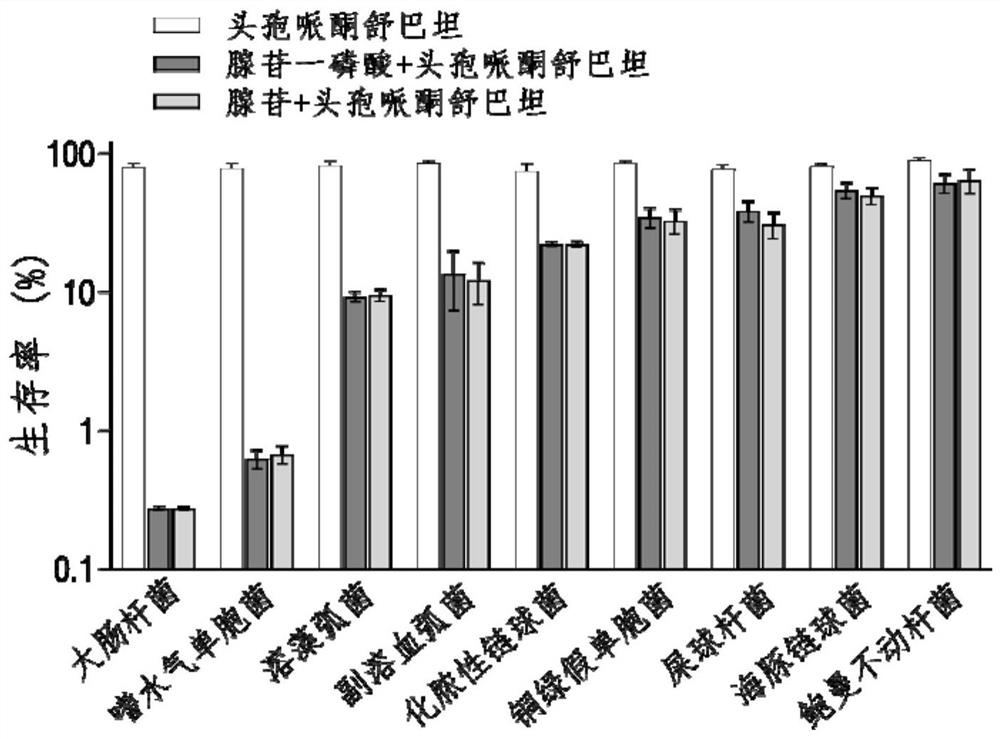
The invention belongs to the technical field of medicine, and particularly relates to application of hypoxanthine nucleotide in preparation of anti-infective drugs. Experiments prove that the hypoxanthine nucleotide can significantly improve the sensitivity of Escherichia coli, Klebsiella pneumoniae, staphylococcus aureus multi-drug-resistant bacteria, pseudomonas aeruginosa and other bacteria toamoxicillin, cefoperazone, meropenem, gentamicin and other antibiotics, so that the hypoxanthine nucleotide can be used as an anti-infective drug together with antibiotics. Bacteria are killed under the condition of low-concentration antibiotics, a good anti-infection effect is achieved, and meanwhile bacterial drug resistance is reduced.
Owner:SUN YAT SEN UNIV
Cefoperazone sodium tazobactam sodium medicine composition for injection and preparing process of cefoperazone sodium tazobactam sodium medicine composition
ActiveCN104958302AReduce generationAvoid it happening againAntibacterial agentsHeterocyclic compound active ingredientsDrugs preparationsAnaphylactic reactions
The invention relates to the field of medicine preparations, and in particular to a cefoperazone sodium tazobactam sodium medicine composition for injection and a preparing process of the cefoperazone sodium tazobactam sodium medicine composition. The cefoperazone sodium tazobactam sodium medicine composition for injection comprises cefoperazone, tazobactam and sodium bicarbonate, and the weight ratio of the cefoperazone to the tazobactam to the sodium bicarbonate is 8:2:1.52-1.68. By means of the medicine composition, the clinic safety performance is improved, and risks of anaphylactic reactions are reduced; and meanwhile the product quality is guaranteed. The invention further provides the preparing process of the medicine composition. The process is simple, the cost is saved, the mixing uniformity of the medicine composition can be effectively improved, generation of polymer in the preparing process is effectively reduced, the polymer and other impurities generated by the high temperature in the split packaging process are prevented, and meanwhile oxidation of active ingredients in the preparing process is reduced.
Owner:JINAN KANGHE MEDICAL TECH
Processing technology for Cefoperazone sodium tazobactam sodium for injection
InactiveCN104382907AReduce labor intensitySimple processing technologyAntibacterial agentsPowder deliveryBiochemical engineeringProcess engineering
The invention discloses a processing technology for cefoperazone sodium tazobactam sodium for injection. The processing technology includes: firstly drying cefoperazone sodium raw material and tazobactam sodium raw material, then conducting powder mixing, finally subpackaging the well mixed cefoperazone sodium and tazobactam sodium antibiotic with well prepared antibiotic bottles, carrying out cover pressing treatment, and then performing light inspection packaging. The processing process is simple and is low in cost, can effectively save time and economic cost, and can reduce the labor intensity of workers.
Owner:SICHUAN PHARMA
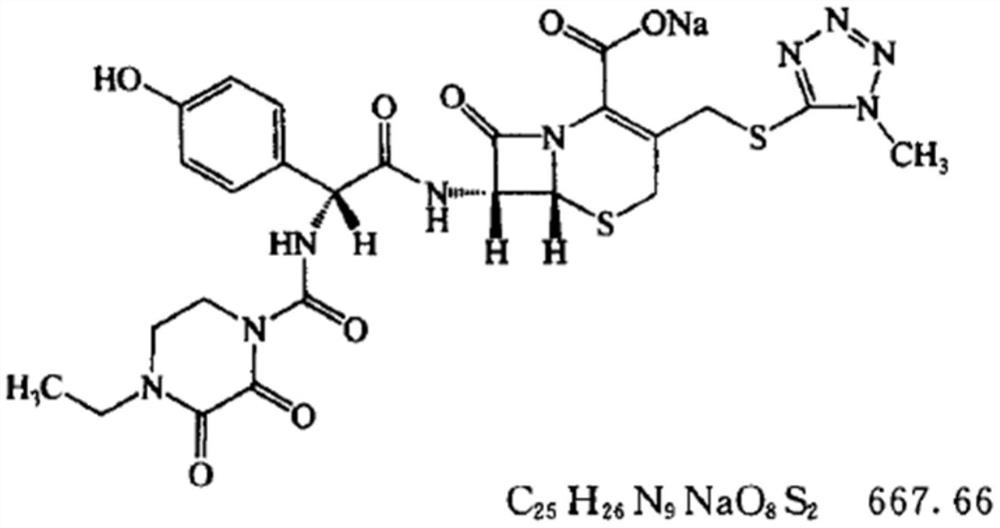
Cefoperazone sodium compound and sulbactam sodium compound prepared with strong-field coupling crystallization technology as well as prepared composition
InactiveCN106432277AHigh purityImprove stabilityAntibacterial agentsPowder deliveryPhysical chemistryPharmaceutical Substances
The invention discloses a cefoperazone sodium compound and a sulbactam sodium compound. The cefoperazone sodium compound and the sulbactam sodium compound are both prepared with a strong-field coupling crystallization technology, specifically, ultrasonic out-field strengthened crystals are added in the compound crystallization process, and the high-purity compounds are prepared. 'Research & Development of the Industrialization Project of High-end Medicine Product Refining Crystallization Technologies' wins the 2015 national scientific and technological progress second prize, and the high-field coupling crystallization technology belongs to one of high-end medicine product refining crystallization technologies. The two compounds have the characteristics of high purity and good stability. Besides, the invention further discloses cefoperazone sodium and sulbactam sodium medicinal composition. The cefoperazone sodium and sulbactam sodium medicinal composition is prepared from components in parts by weight as follows: 0.25-2 parts of cefoperazone sodium (on the basis of C25H27N9O8S2) and 0.25-2 parts of sulbactam sodium (on the basis of C8H11NO5S).
Owner:陕西顿斯制药有限公司
Oral preparation containing cefoperazone schubatain and its making method
An orally absorbed medicine in the form of buccal lozenge, sweets, or dripping pill dissolved by saliva features that it contains cefoperazone and sulbactam. Its preparing process is also disclosed.
Owner:刘凤鸣
Composition of cefperazone sodium and sulbactam sodium and lysine
ActiveCN101780086AGood kidney safetyImprove stabilityAntibacterial agentsPowder deliveryInfective disorderSulbactam Sodium
The invention belongs to the technical field of medicine, in particular relates to a composition of cefperazone sodium and sulbactam sodium and lysine, and also provides a preparation method of the composition injection and an application of the composition for preparing drugs of the infectious diseases caused by beta-lactamase bacteria which resists cefoperazone but is sensitive to the composition.
Owner:BEIJING SIHUAN PHARMA +1
Powder injection composed of sodium cefoperazone and potassium clavulanate
A powder injection for treating bacterium-infectious diseases is prepared from cefoperazone sodium and potassium clavulanate in weight ratio of (1-16):1.
Owner:苏大阳
Cefoperazone sodium and sulbactam sodium powder for injection and production method of cefoperazone sodium and sulbactam sodium powder for injection
PendingCN110893232AImprove stabilityImprove color levelAntibacterial agentsPowder deliveryDisodium phosphateSodium phosphates
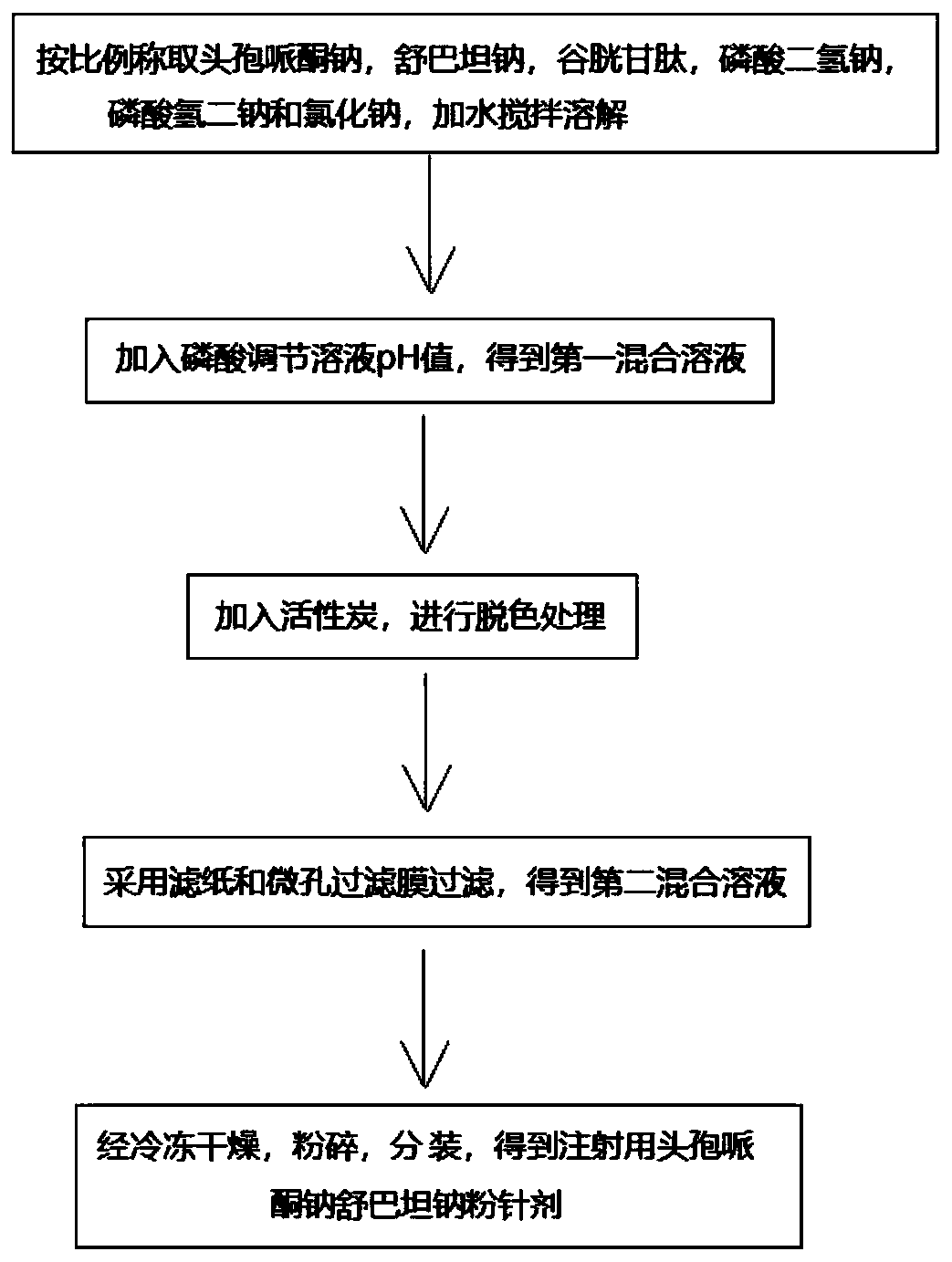
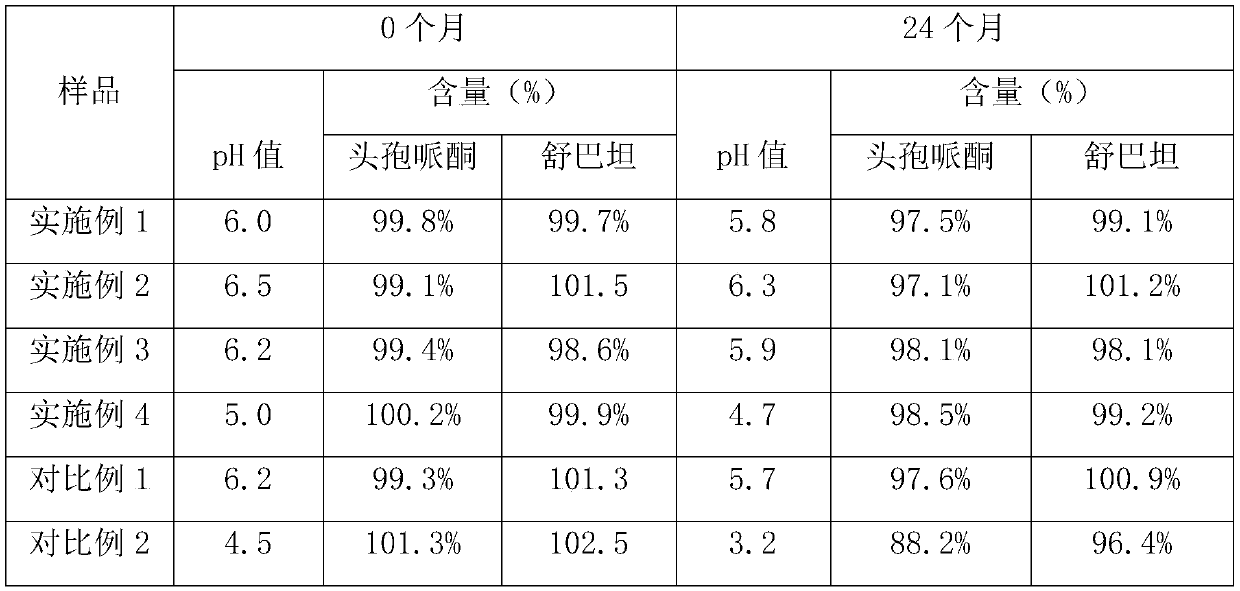
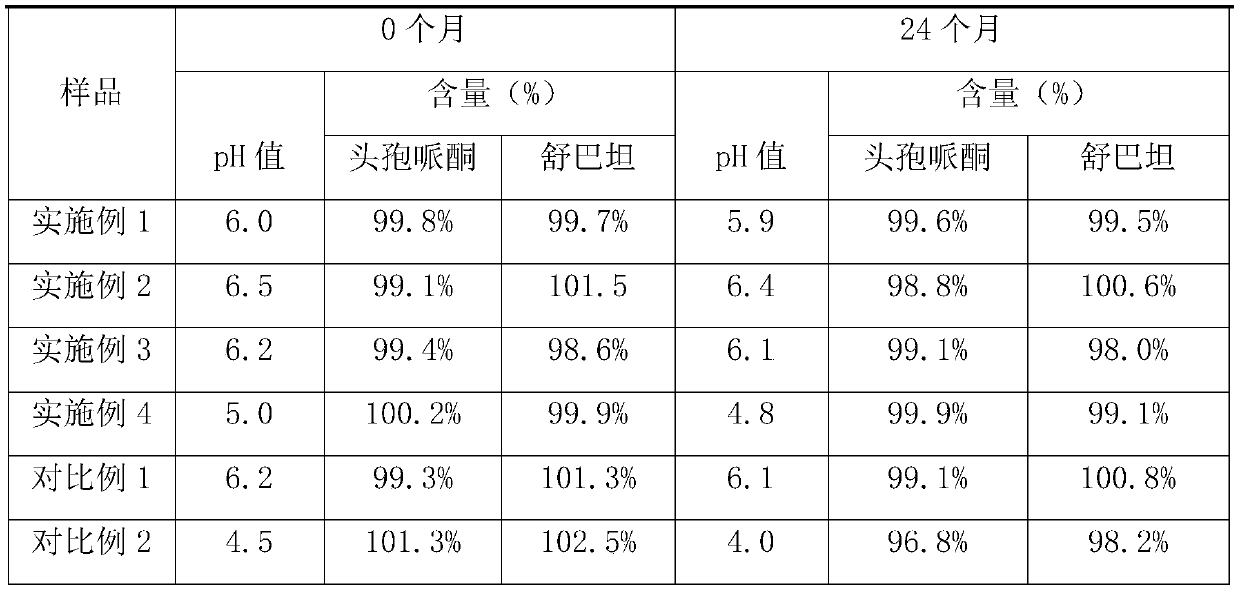
The invention discloses cefoperazone sodium and sulbactam sodium powder for injection. The cefoperazone sodium and sulbactam sodium powder for injection comprises the following components in parts bymass: 10-26 parts of cefoperazone sodium, 5-12 parts of sulbactam sodium, 0.5-1.2 parts of glutathione, 0.1-0.6 part of sodium phosphate monobasic, 0.1-1.5 parts of disodium hydrogen phosphate and 0.3-0.8 part of sodium chloride, and a pH value of the powder for injection is 5.0-6.5. The cefoperazone sodium and sulbactam sodium powder for injection has excellent stability and an excellent anti-bacterial effect, and the safety is high.
Owner:上海欣峰制药有限公司
Preparation method of cefoperazone sodium
ActiveCN112279867ALow impurity contentUniform particlesOrganic chemistrySodium bicarbonateNitrogen gas
The invention discloses a preparation method of cefoperazone sodium, which comprises the steps of S1, adding acetone into a reaction tank, stirring to control the temperature, and adding cefoperazoneacid to obtain a cefoperazone acid solution; S2, adding purified water and sodium bicarbonate into a dissolving tank, stirring and controlling the temperature to obtain alkali liquor; S3, feeding thealkali liquor in the step S2 into a cefoperazone acid solution, controlling the temperature, and pressing into a crystallizing tank through a degerming and filtering line; S4, respectively introducingsterile nitrogen and acetone into the crystallizing tank through a gas-liquid two-phase nozzle; S5, adding seed crystal, stirring and growing crystal; S6, continuously adding nitrogen and acetone through the gas-liquid two-phase nozzle; and S7, after feeding is completed, conducting cooling, crystal growing, filtering, vacuum drying and discharging. The cefoperazone sodium powder prepared throughthe method is uniform in particle, small in specific volume and low in acetone solvent residue.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for determining content of cefoperazone sodium of cefoperazone sodium and tazobactam sodium for injection
InactiveCN104198549ASimple methodFast wayPreparing sample for investigationMaterial electrochemical variablesDifferential pulse voltammetryAnalytical chemistry
The invention discloses a method for determining the content of cefoperazone sodium of cefoperazone sodium and tazobactam sodium for injection. A differential pulse voltammetry is adopted to scan the cefoperazone sodium and tazobactam sodium and can accurately quantify the cefoperazone sodium by the obtained peak current value. The method is simple and rapid and can achieve ideal sensitivity and accuracy.
Owner:SICHUAN PHARMA
Application of beta-lactam compounds
InactiveCN111184726AInhibition of metastatic progressionOrganic active ingredientsAntineoplastic agentsChemical compoundEnzyme binding
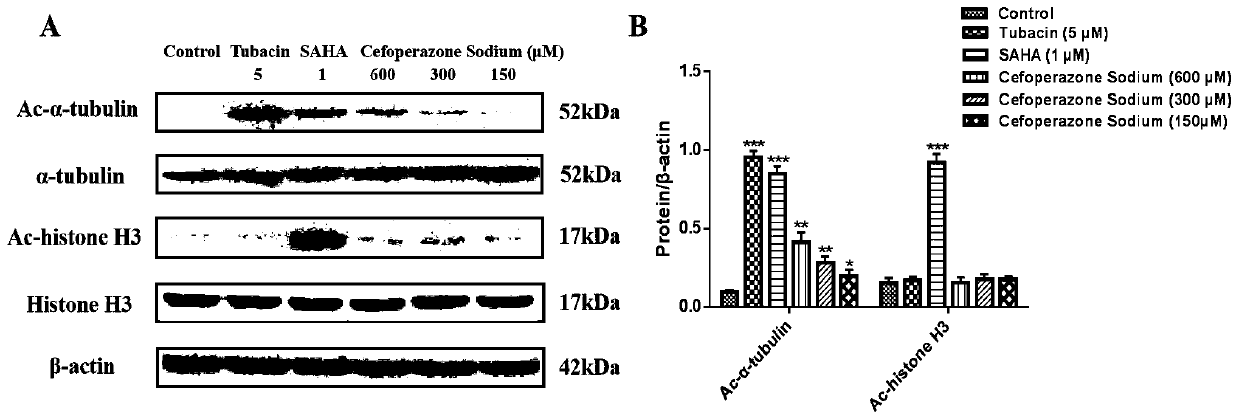
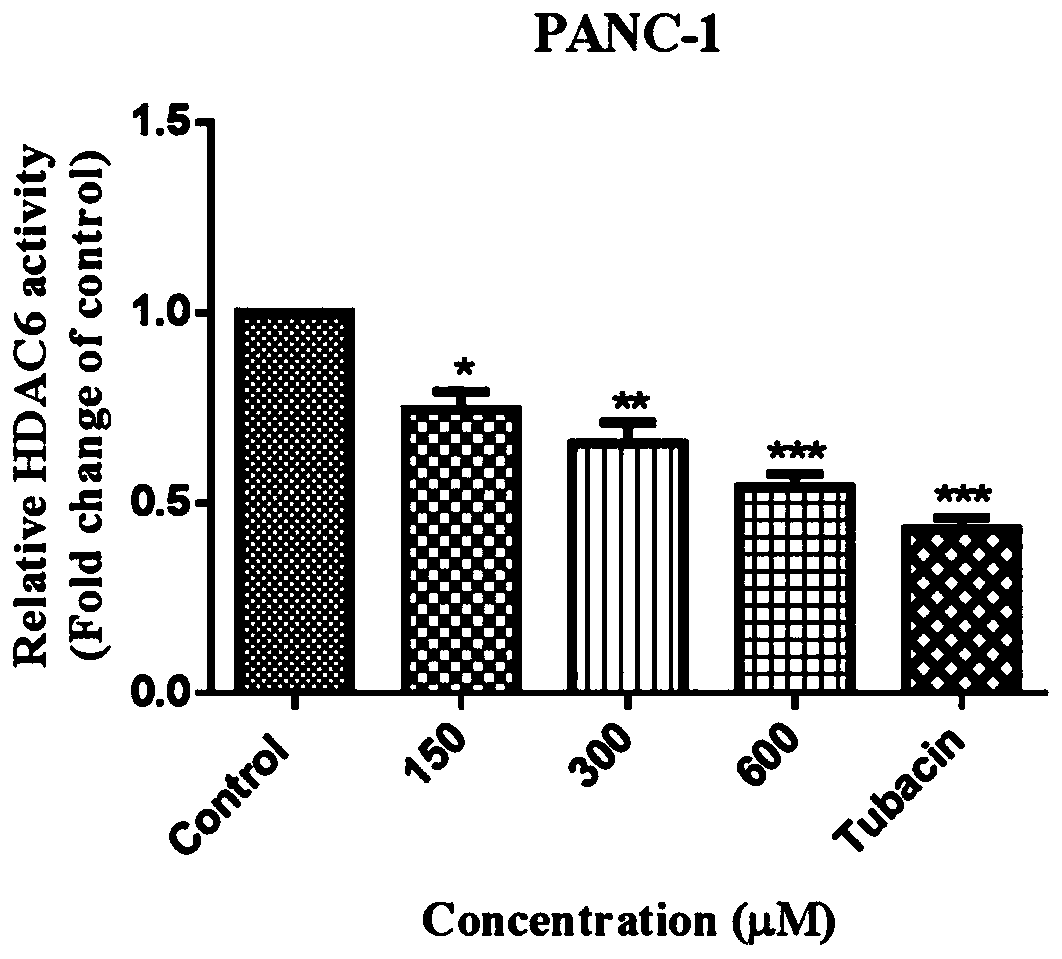
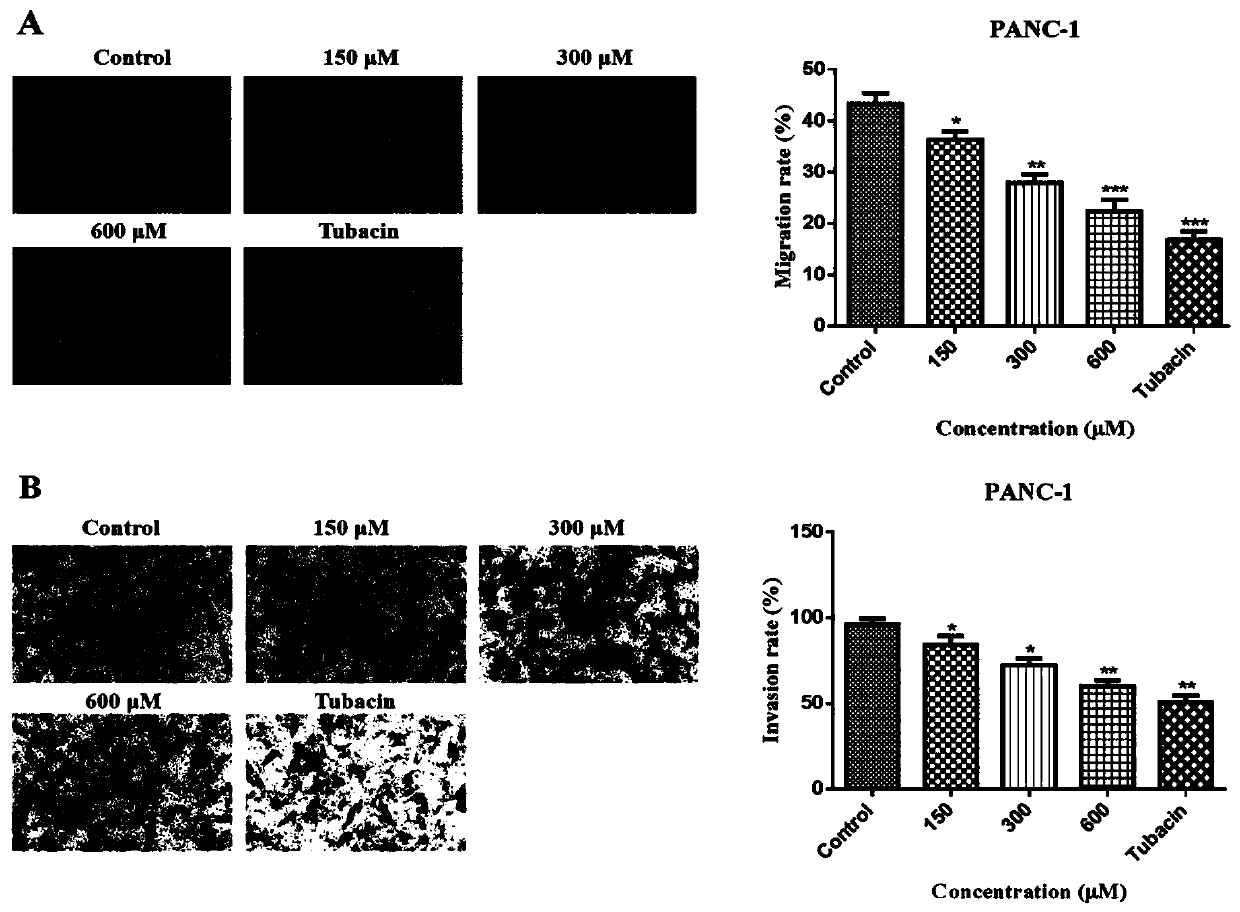
The invention belongs to the technical field of medicines, and relates to application of beta-lactam compounds, in particular to application of beta-lactam compounds in production of histone deacetylase inhibitors and application of the beta-lactam compounds in production of anti-tumor-metastasis medicines. According to application of the beta-lactam compounds in production of the histone deacetylase inhibitors and application of the beta-lactam compounds in production of the anti-tumor-metastasis medicines, it is found by research through a substrate-enzyme binding test, immunoblotting, cellenzyme activity detection, a scratch test and a Transwell test that cefoperazone has a significant inhibiting effect on malignant tumor metastasis by inhibiting the activity of HDAC6. Therefore, the beta-lactam compounds represented by the cefoperazone can be used for production of the anti-tumor-metastasis medicines, and have excellent application prospects in prevention and inhibition of postoperative metastasis of malignant tumors.
Owner:SHENYANG PHARMA UNIVERSITY
Application of adenosine or adenosine monophosphate in preparation of anti-infective drugs
PendingCN114159456AIncreased sensitivityDelay drug resistanceAntibacterial agentsOrganic active ingredientsMeropenemVibrio parahaemolyticus
The invention belongs to the technical field of biological medicine, and particularly relates to application of adenosine or adenosine monophosphate in preparation of anti-infective drugs. The invention relates to a preparation method of the medicine. Adenosine or adenosine monophosphate can significantly improve the effect of clinical escherichia coli, aeromonas hydrophila, vibrio including vibrio alginolyticus and vibrio parahaemolyticus, streptococcus pyogenes, pseudomonas aeruginosa, bacillus faecium, streptococcus iniae, acinetobacter baumannii, klebsiella pneumoniae and other bacteria on the effects of cefoperazone sulbactam, ceftazidime, ceftriaxone sodium, ceftazidime, ceftriaxone sodium, ceftazidime, ceftriaxone sodium, ceftazidime sodium and the like. The sensitivity of antibiotics such as cefoperazone, meropenem, imipenem, ciprofloxacin, ampenem, moxifloxacin, levofloxacin, gentamicin, amikacin, kanamycin and the like is improved, the compound can be used together with the antibiotics to serve as an anti-infection drug, bacteria are killed under the condition of low-concentration antibiotics, and a good anti-infection effect is achieved; meanwhile, the generation of bacterial drug resistance is reduced.
Owner:SUN YAT SEN UNIV
Cefoperazone sodium sulbactam sodium composition pharmaceutical preparation and new indications for treating infective endocarditis
ActiveCN110393719BIncrease contentLess impuritiesAntibacterial agentsOrganic active ingredientsEndocarditisInfective endocarditis
The invention discloses a new indication for treating infective endocarditis with a pharmaceutical preparation of cefoperazone sodium and sulbactam sodium. The cefoperazone sodium provided by the invention through a specific raw material production process has extremely low impurity content and remarkable medicinal effect, which is conducive to improving the quality of the preparation product, ensures the safety and effectiveness of the preparation product, and has the purposes of preparing a medicine for treating infective endocarditis .
Owner:广东金城金素制药有限公司
Cefoperazone sodium for injection and preparation method thereof
ActiveCN110507619BQuality assuranceImprove efficiencyAntibacterial agentsOrganic active ingredientsClinical efficacyBiochemistry
The invention relates to cefoperazone sodium for injection and a preparation method thereof, and belongs to the technical field of medicine. The cefoperazone sodium for injection is prepared by adding 500g to 2000g of cefoperazone sodium, sodium citrate, povidone K30, adding 2000ml of water for injection and then freeze-drying. The cefoperazone sodium for injection obtained by the invention has good quality and high stability, is superior to the prior art, and greatly improves the effectiveness and safety of the cefoperazone sodium for injection in clinical use.
Owner:湖北美林药业有限公司
Composition containing piperacillin, its pharmaceutical preparation and its application
ActiveCN107875154BPlay a therapeutic effectAntibacterial agentsHeterocyclic compound active ingredientsDrugs preparationsTherapeutic effect
The invention provides a piperacillin-containing composition. The composition contains piperacillin and a certain proportion of ampicillin and sulbactam. The invention also provides its pharmaceutical preparation and application. Compositions and pharmaceutical preparations of the present invention can significantly inhibit drug-resistant Acinetobacter baumannii, especially to carbapenem antibiotics or cefoperazone-sulbactam-resistant Acinetobacter baumannii infection can play a significant role. Good therapeutic effect, with significant clinical advantages.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Medicine formula for treating prostatitis and benign prostatic hyperplasia and preparation process thereof
PendingCN113662947AGuaranteed to be carried out effectivelyEnsure safetyAntibacterial agentsUrinary disorderProcaineFormulary
The invention discloses a medicine formula for treating prostatitis and benign prostatic hyperplasia and a preparation process thereof. The medicine formula comprises the following components by weight: 1000 to 8000 mg of cefoperazone sodium sulbactam sodium, 2 to 40 mg of edetate disodium, 1 to 20 mg of dexamethasone, 20 to 200 mg of levobupivacaine, and 40 to 400 mg of procaine. The compatible formula is directly administered through prostate microneedle injection, so that the effects of resisting bacteria, diminishing inflammation and relieving prostatic fibrosis in the prostate body are achieved to the maximum extent, and the formula is suitable for treating various chronic prostatitis and benign prostatic hyperplasia. A large number of clinical cases prove that the formula has the advantages of remarkable curative effect, no pain in treatment, safety, no side effect and the like.
Owner:查国章 +4
An indirect competition ELISA kit for detecting cephalosporin antibiotics in food of animal origin and its application
ActiveCN107014993BIncreased cross-reactivityIncreased sensitivityMaterial analysisElisa kitCefotaxime
The invention discloses an indirect-competitive ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting cephalosporin antibiotics in animal derived foods and application of the kit. The kit comprises an ELISA plate coated with a coating antigen, a cephalosporin antibiotic standard substance, a cephalosporin antibiotic general antibody, an enzyme labeled secondary antibody, a dilution buffer, a washing buffer, a substrate developing solution and a stop solution, wherein the cephalosporin antibiotic general antibody is capable of specifically identifying cefalexin, cefradine, cefadroxil, cefoperazone, cefazolin or cefotaxime and cannot identify penicillin sodium. According to the cephalosporin antibiotic general antibody prepared in the invention, the general antibody and most of cephalosporin antibiotics have high cross reaction rates, while hardly have any cross reaction for the analogue penicillin sodium containing a beta-lactam ring; and therefore, the general antibody has high specificity. The kit prepared by the general antibody has the advantages of high sensitivity, capability of detecting many types of drugs, low cost, simple operation and short detection time.
Owner:HEBEI AGRICULTURAL UNIV.
Method for determining contents of effective components in cefoperazone sodium and tazobactam sodium for injection
InactiveCN104165919AThe detection method is simple and fastLow costMaterial analysis by electric/magnetic meansElectrophoresesHydrochlorothiazide
The invention discloses a method for determining contents of effective components in cefoperazone sodium and tazobactam sodium for injection. According to the method, a capillary electrophoresis method is used, and hydrochlorothiazide is taken as an interior label for quantitatively determining cefoperazone sodium and tazobactam sodium. The method for determining contents of effective components in cefoperazone sodium and tazobactam sodium for injection is relatively simple and quick, low in cost and capable of effectively controlling quality in the process of producing cefoperazone sodium and tazobactam sodium preparations for injection.
Owner:SICHUAN PHARMA
Method for determining cefoperazone sodium and sulbactam sodium related substances for injection
ActiveCN112415112AIncrease temperatureDissolve fastComponent separationRotary stirring mixersDrug productSulbactam Sodium
The invention discloses a method for determining cefoperazone sodium and sulbactam sodium related substances for injection. The method comprises the following steps: preparing a solution by using solution preparation equipment, adding cefoperazone sodium and sulbactam sodium powder into a mixing tank through a feeding hopper, introducing water into the mixing tank, closing the feeding hopper, andstarting a driving mechanism and a heating mechanism. According to the method for determining the cefoperazone sodium and sulbactam sodium related substances for injection, the cefoperazone sodium andsulbactam sodium powder can be rapidly dissolved through forward and reverse rotation of a stirring frame I and a stirring frame II in the mixing tank, the temperature of the solution is increased bythe heating mechanism, and the dissolving efficiency of medicine powder is further improved; the dissolving effect of the cefoperazone sodium and sulbactam sodium powder is effectively improved, 14 impurities in the cefoperazone sodium and sulbactam sodium for injection can be effectively separated and quantitatively determined by adopting a newly established RPHPLC method, the system applicability of chromatography is high, the product quality can be more effectively controlled, and safe popularization and application are facilitated.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Purification method of cefoperazone sodium
ActiveCN112279868AQuality improvementLow impurity contentOrganic chemistrySodium bicarbonateActivated carbon
The invention discloses a purification method of cefoperazone sodium, which comprises the steps of S1, adding a cefoperazone sodium and sulbactam sodium product into purified water, stirring until thesolution is clear, controlling the temperature, adding acetone into the reaction tank, and starting stirring until the cefoperazone sodium and sulbactam sodium are completely dissolved to obtain a dissolved solution; S2, adding activated carbon into the dissolved solution for decoloration, and filtering to obtain a destaining solution for later use; S3, adding acetone, isopropanol and ethanol into a crystallizer to obtain a mixed solution, and controlling the temperature of the crystallizer; S4, adding the destaining solution obtained in the step S2 into the crystallizer in a rapid stirring state, then dropwise adding a sodium bicarbonate solution to adjust the pH value of the feed liquid, and growing crystals; S5, after crystal growing is finished, cooling for crystal growing, filtering,and washing with an acetone-water mixed solution; and S6, conducting vacuum drying and discharging. The prepared cefoperazone sodium has the advantages of being stable in quality, low in impurity content and high in product content.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
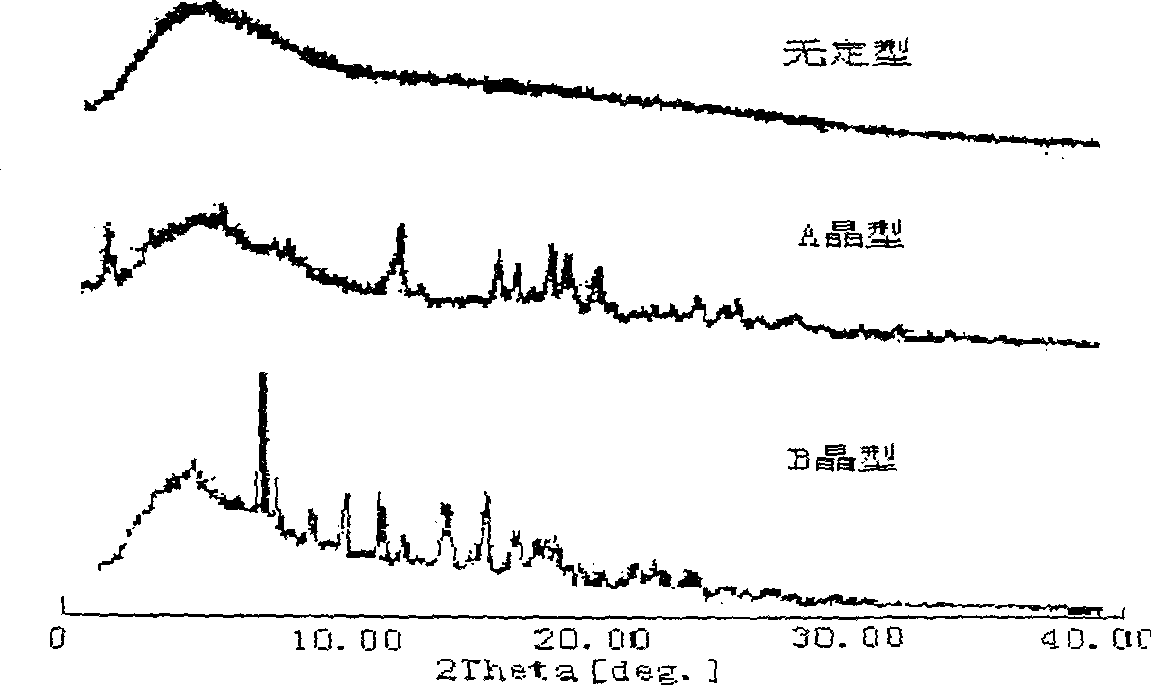
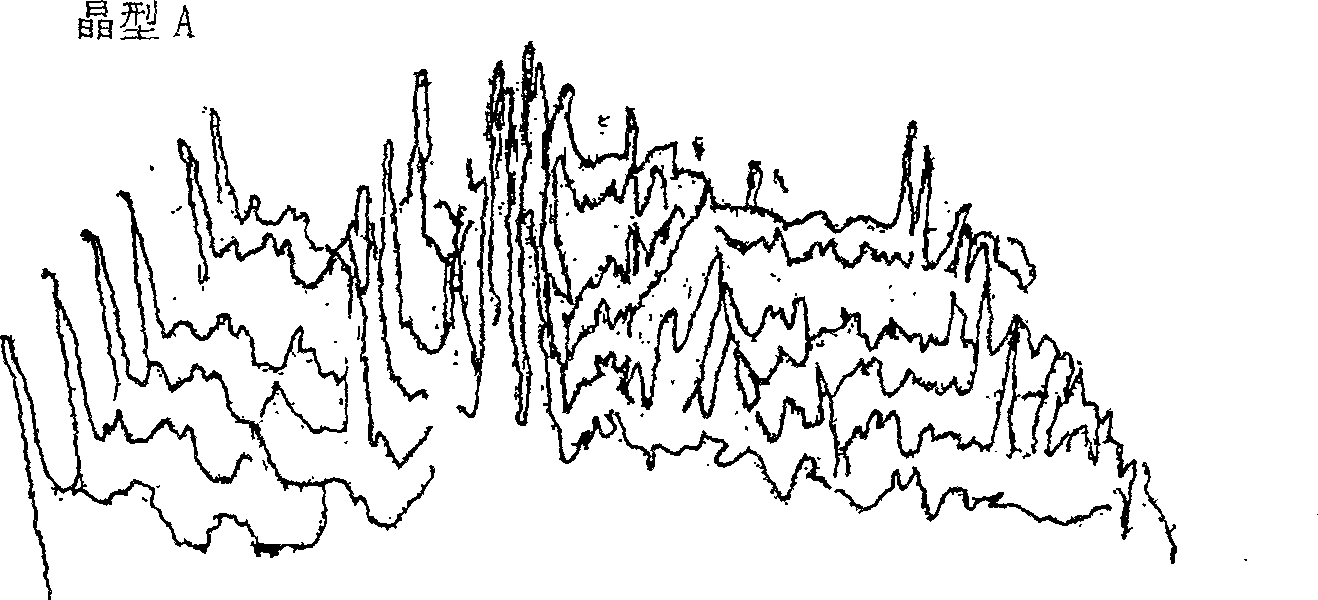
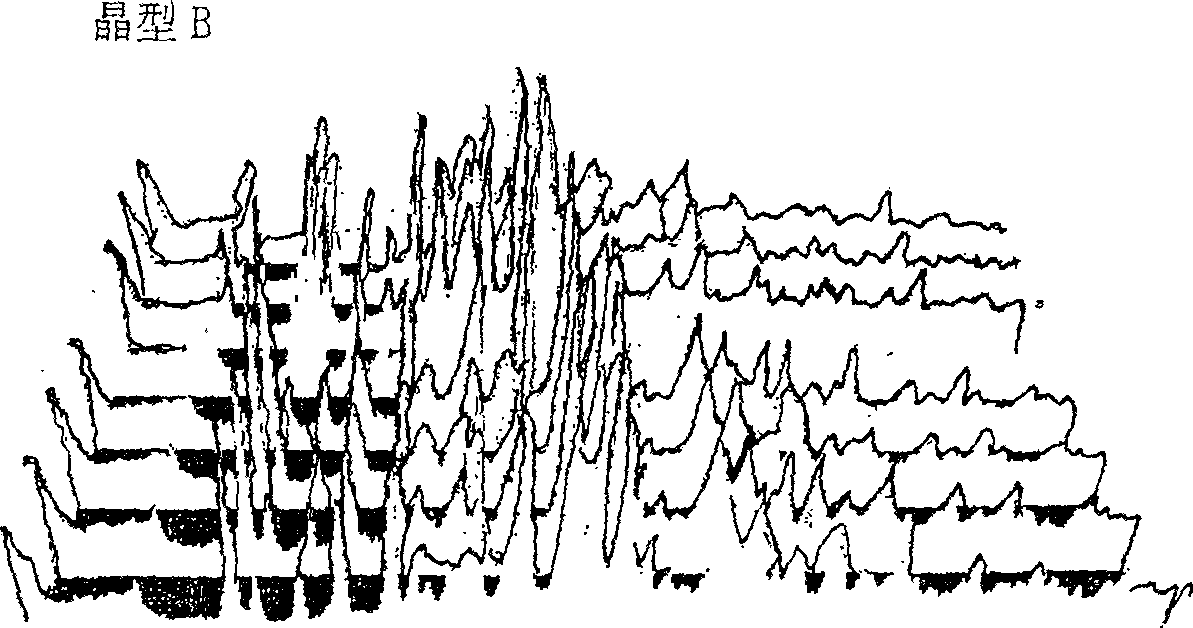
Cefoperazone sodium novel crystal form and its preparation method
ActiveCN100532383CImprove stabilityOrganic chemistryPharmaceutical delivery mechanismPharmaceutical drugBiochemistry
The invention provides a stable new crystal form of cefoperazone sodium, which is composed of one or more of subtypes I, II and III of crystal form A, and the invention also provides a preparation method of the three subtypes, And the application of these three subtypes in the preparation of anti-infective drugs. The crystal form of the present invention has better stability than the crystal form of the prior art.
Owner:SHANGHAI NEW ASIA PHARMA +1
Composition for treating prostatitis and benign prostatic hyperplasia and preparation method thereof
InactiveCN113995759APromote absorptionTo promote metabolismOrganic active ingredientsPharmaceutical delivery mechanismDisodium EdetateAntimicrobial drug
The invention relates to a composition for treating prostatitis and benign prostatic hyperplasia and a preparation method thereof. The composition is prepared from the following raw materials in mass ratio: 1.0-8.0 g of cefoperazone sodium sulbactam sodium, 2-40 mg of edetate disodium, 100-600 mg of reduced glutathione for injection and 20-200 mg of lidocaine. Compared with a conventional oral administration, intramuscular injection or intravenous drip medicine treatment method, the composition prepared by the method has the following advantages: the medicine directly acts on an infected part, and the lasting time of the medicine effect is long; the treatment process is easy, an operation is not needed, tissues are not damaged, a patient has no obvious pain, and the patient can freely move after being treated; various adverse reactions such as serious dysfunction of flora in the body of a patient caused by long-term oral administration, intramuscular injection or intravenous drip of antibacterial drugs are avoided.
Owner:刘立鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com