Method for determining cefoperazone sodium and sulbactam sodium related substances for injection
A technology for sofoperazone sodium and sulbactam sodium and related substances is applied in the field of determination of related substances of cefoperazone sodium and sulbactam sodium for injection, which can solve the problems of cumbersome operation process, poor dissolution effect, low dissolution efficiency of cefoperazone sodium and sulbactam sodium, etc. , to achieve the effect of improving the dissolution effect and accelerating the dissolution efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
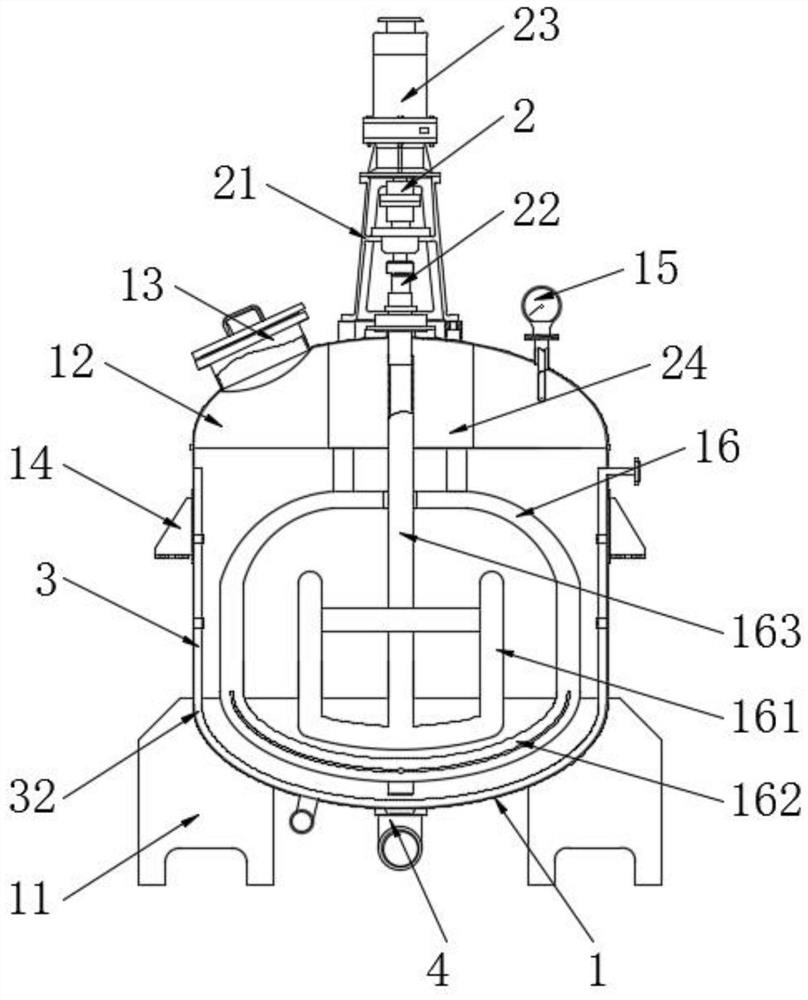
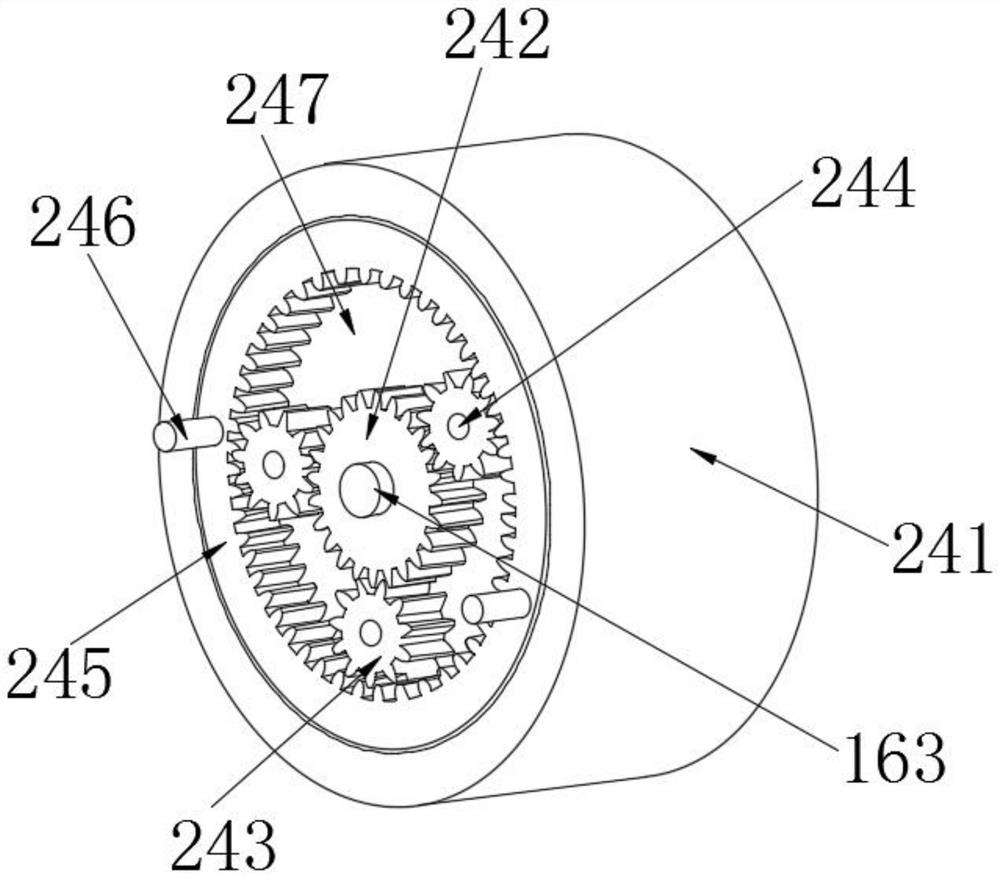
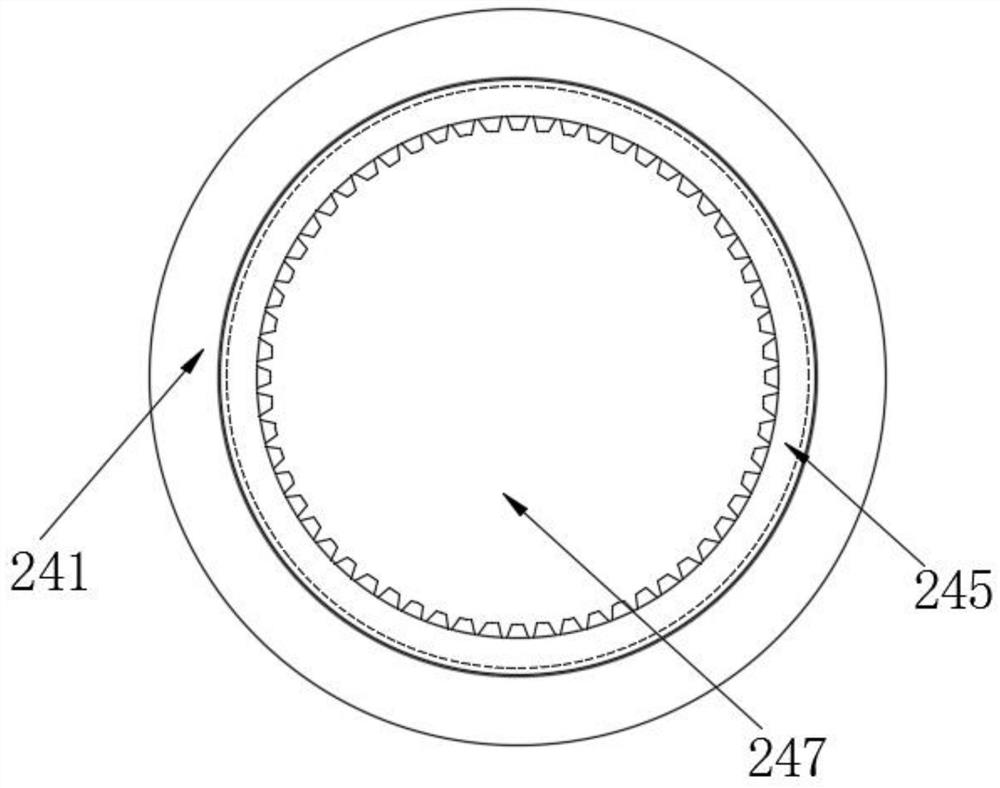
[0031] see Figure 1-5 As shown, the present invention provides a technical scheme: a method for determining related substances of cefoperazone sodium and sulbactam sodium for injection, specifically comprising the following steps:
[0032] Step 1, utilize solution preparation equipment to carry out the preparation of solution, cefoperazone sodium sulbactam sodium powder is dropped in mixing tank 12 through feeding hopper 13, then pass water in mixing tank 12,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com