Cefoperazone sodium and tazobactam sodium medicinal composition microsphere injection
A technology of cefoperazone sodium and tazobactam sodium, which is applied in the field of medicine, can solve the problems of undisclosed cefoperazone sodium and sulbactam sodium, and achieve the effects of good stability, improved stability and high encapsulation efficiency
Inactive Publication Date: 2010-12-15
HAINAN LINGKANG PHARMA CO LTD
View PDF30 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The microsphere preparation of cefoperazone sodium sulbactam sodium is not disclosed in the prior art, and the pharmaceutical composition of excellent cefoperazone sodium sulbactam sodium is still needed in this field, which has excellent slow-release characteristics and stability. The present invention adopts microsphere technology to prepare Cefoperazone sodium and sulbactam sodium microspheres provide microsphere injections of cefoperazone sodium and sulbactam sodium with excellent sustained-release properties and stability
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Login to View More
Abstract
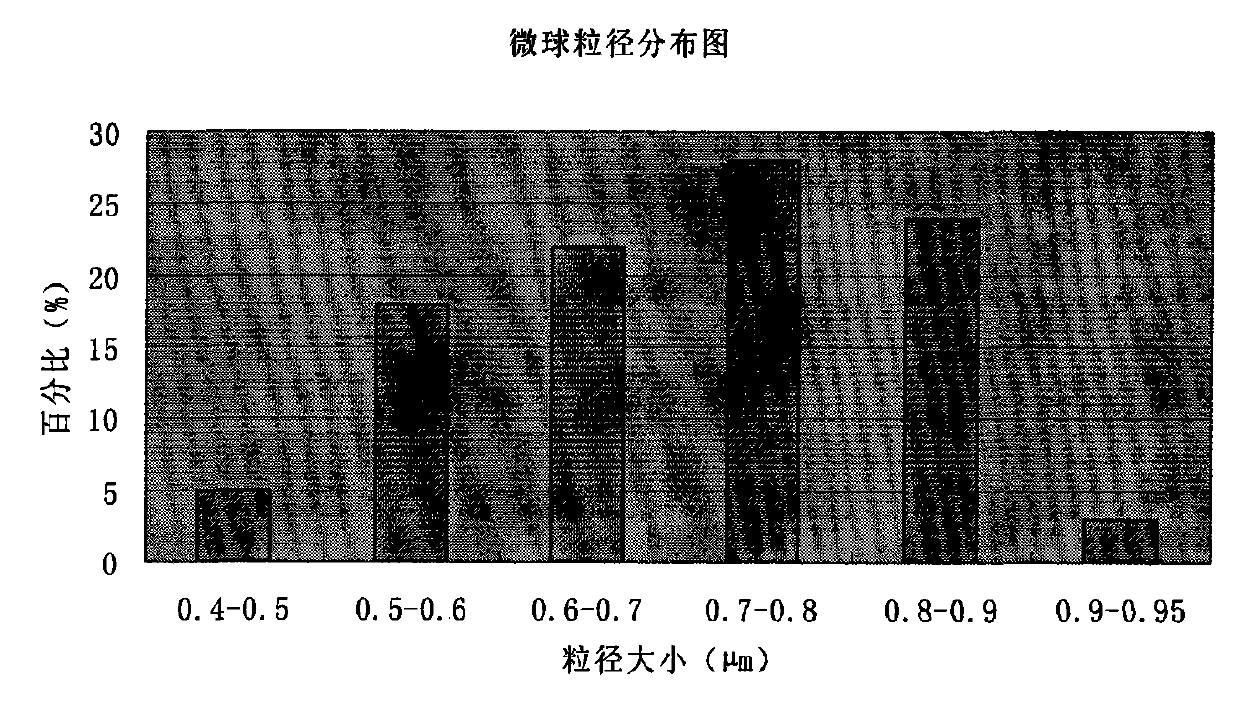
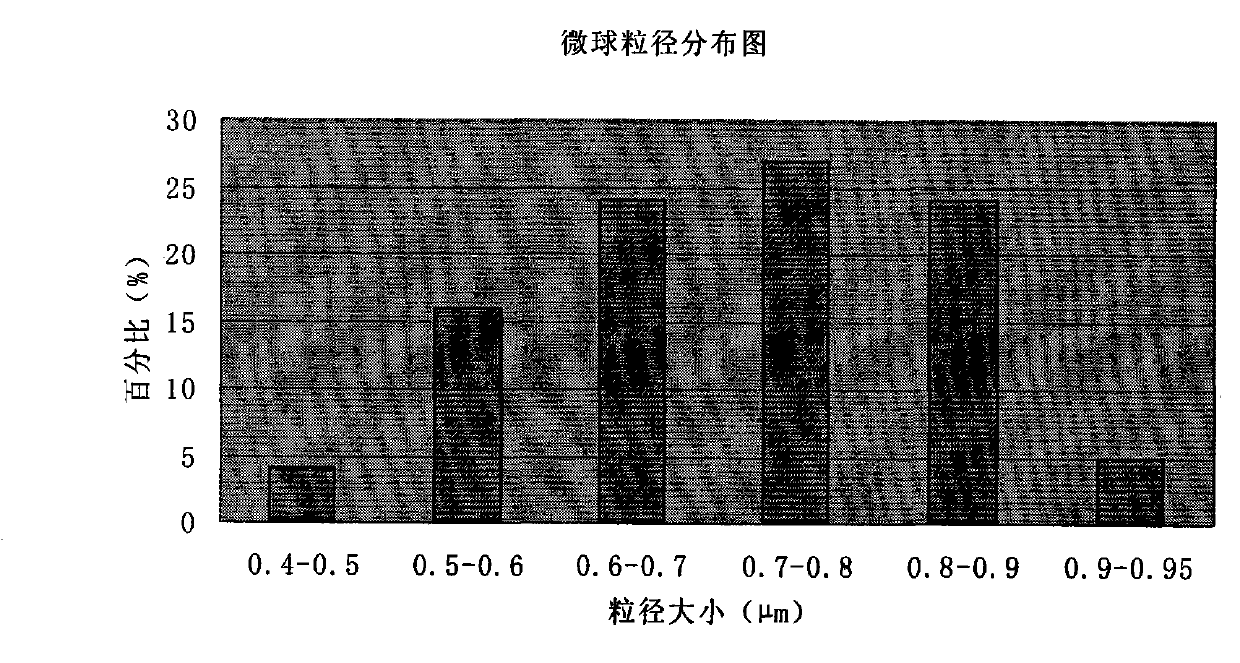
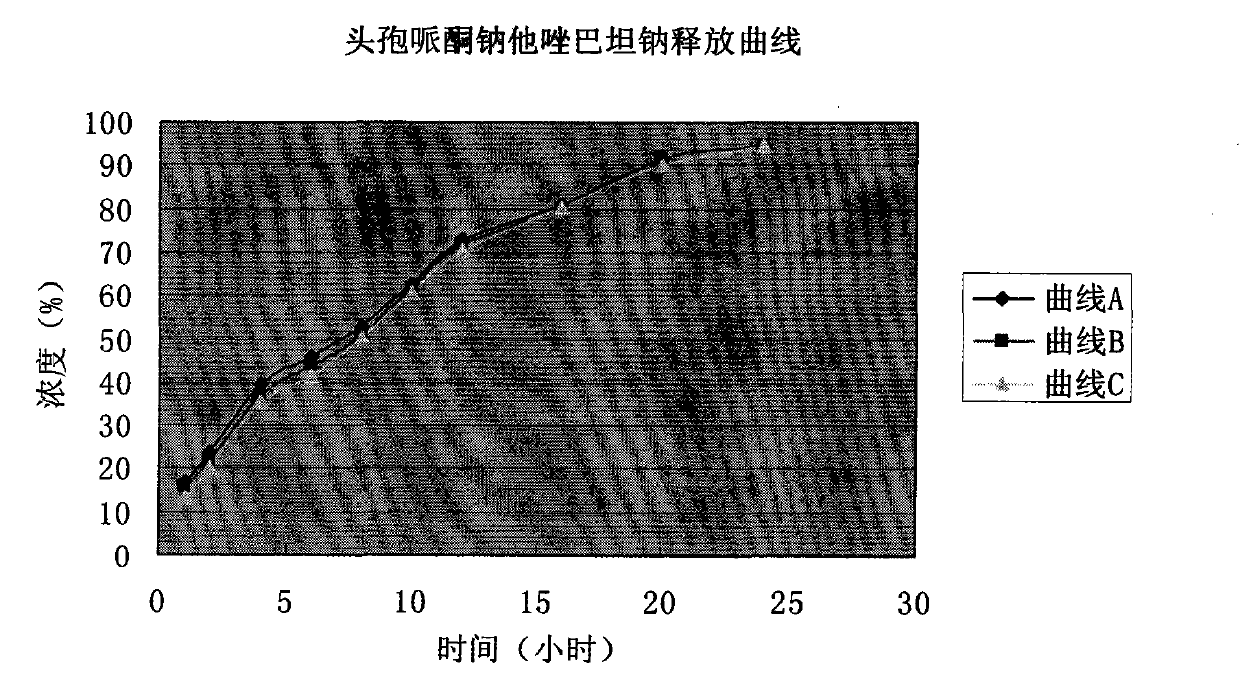
The invention discloses cefoperazone sodium and tazobactam sodium medicinal composition microsphere injection, which is characterized by comprising 4 to 8 weight parts of cefoperazone sodium, 1 to 2 weight parts of tazobactam sodium, 2.5 to 5 weight parts of PLA, 1.25 to 2.5 weight parts of Span60, 1 to 2 weight parts of glycerol monostearate and 1 to 2 weight parts of glucose. Compared with the prior art, the cefoperazone sodium and tazobactam sodium medicinal composition microsphere injection of the invention has the advantages of high stability, high entrapment rate, high-repeatability preparation process suitable for industrial production, uniform particle distribution, little solvent residue, high injectability and good sustained-release property.
Description
technical field The invention relates to a cefoperazone sodium tazobactam sodium pharmaceutical composition microsphere injection, which belongs to the technical field of medicine. Background technique Cefoperazone is a third-generation cephalosporin antibiotic that achieves bactericidal effect by inhibiting the biosynthesis of sensitive bacterial cell walls. Tazobactam has no antibacterial activity against other bacteria except Neisseriaceae and Acinetobacter, but tazobactam has activity against most important β-lactamases produced by β-lactam antibiotic-resistant strains Irreversible inhibition. Tazobactam can prevent drug-resistant bacteria from destroying penicillins and cephalosporins, and tazobactam has obvious synergistic effects with penicillins and cephalosporins. The compound preparation of cefoperazone sodium and tazobactam sodium has been sold in China at present, and all of them are directly packaged as aseptic powder. The common defect is that the preparatio...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/546A61K31/431A61K9/16A61K47/34A61P31/04A61K47/26
Inventor 陶灵刚
Owner HAINAN LINGKANG PHARMA CO LTD
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com