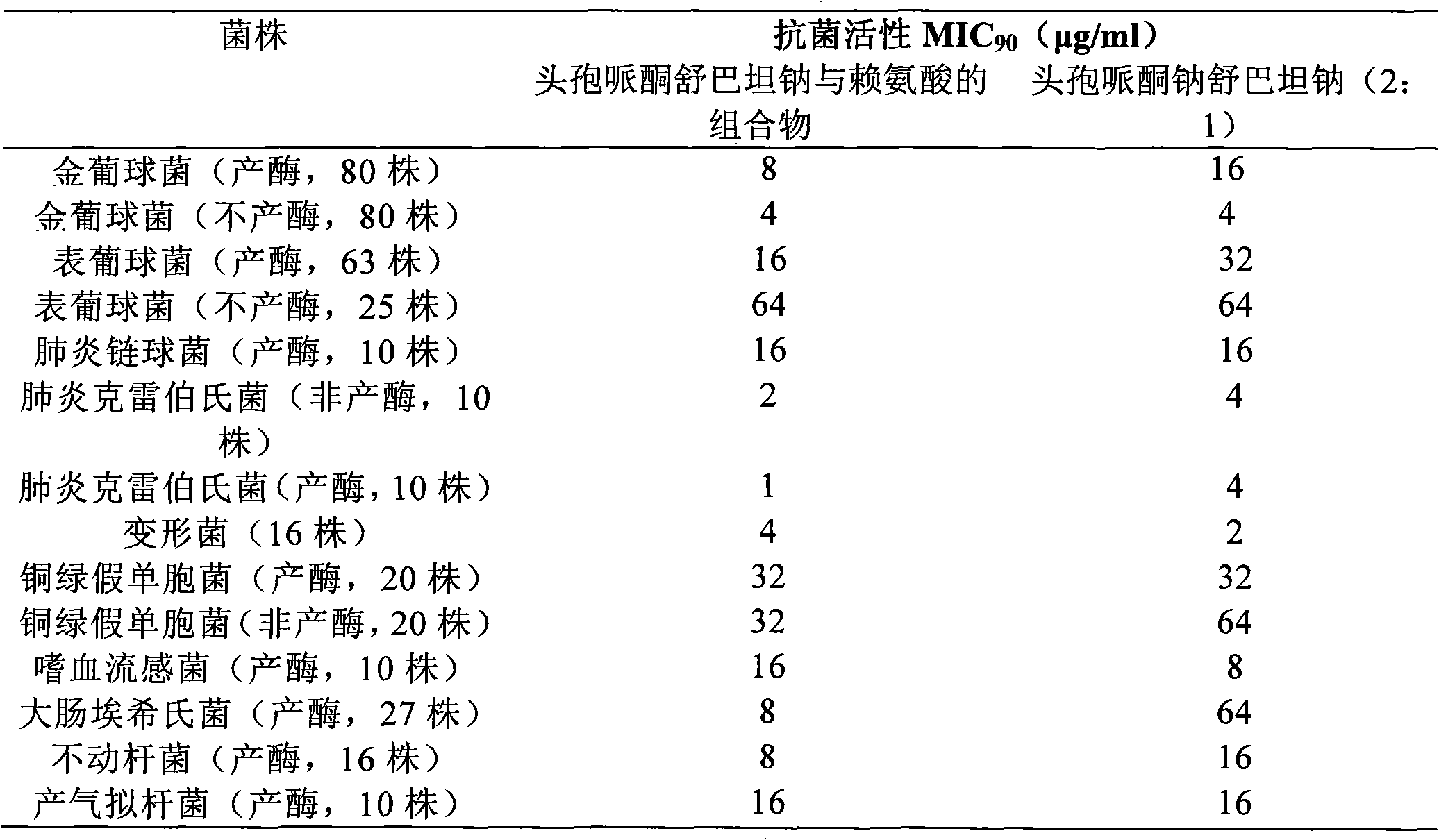
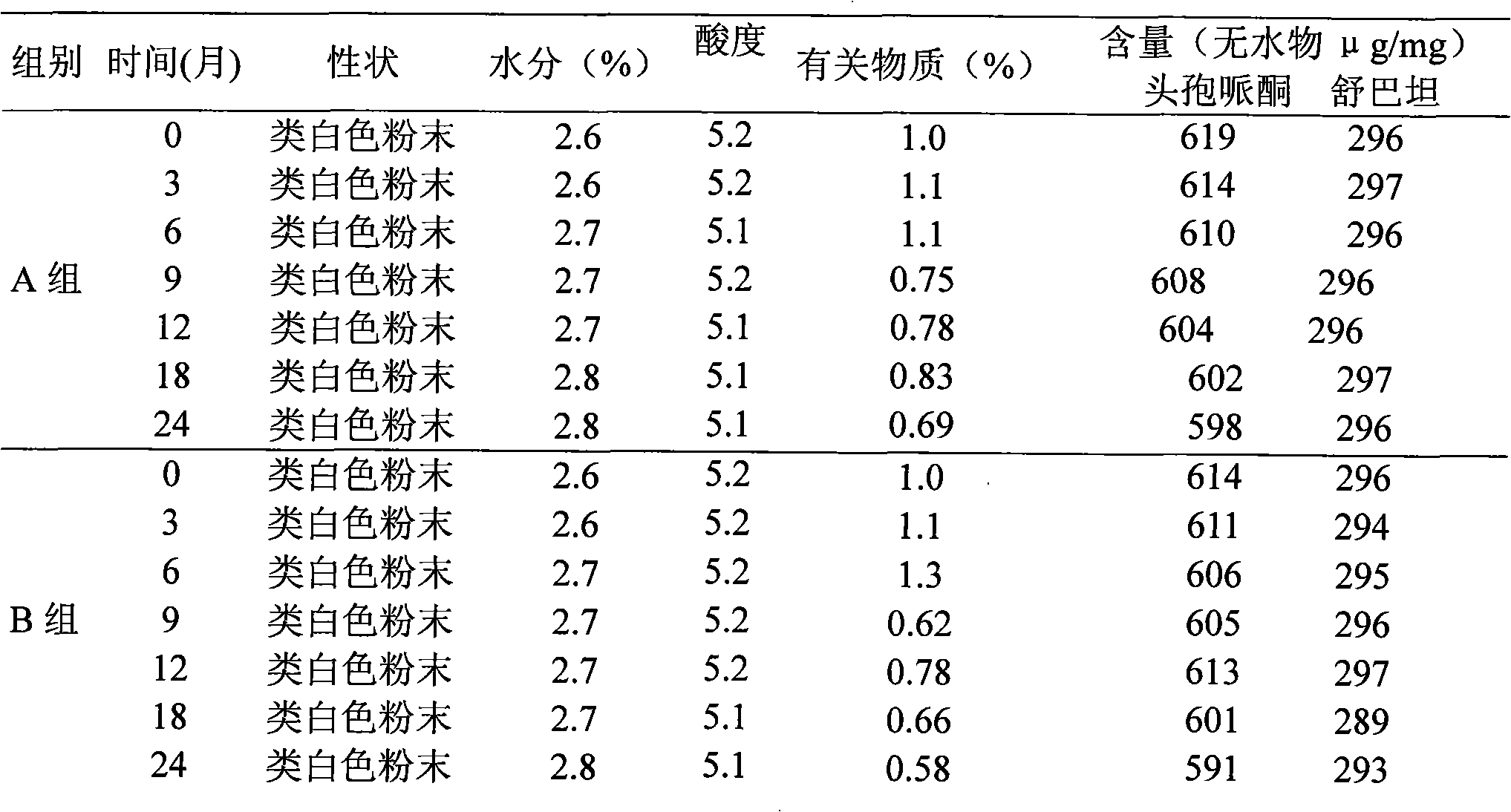
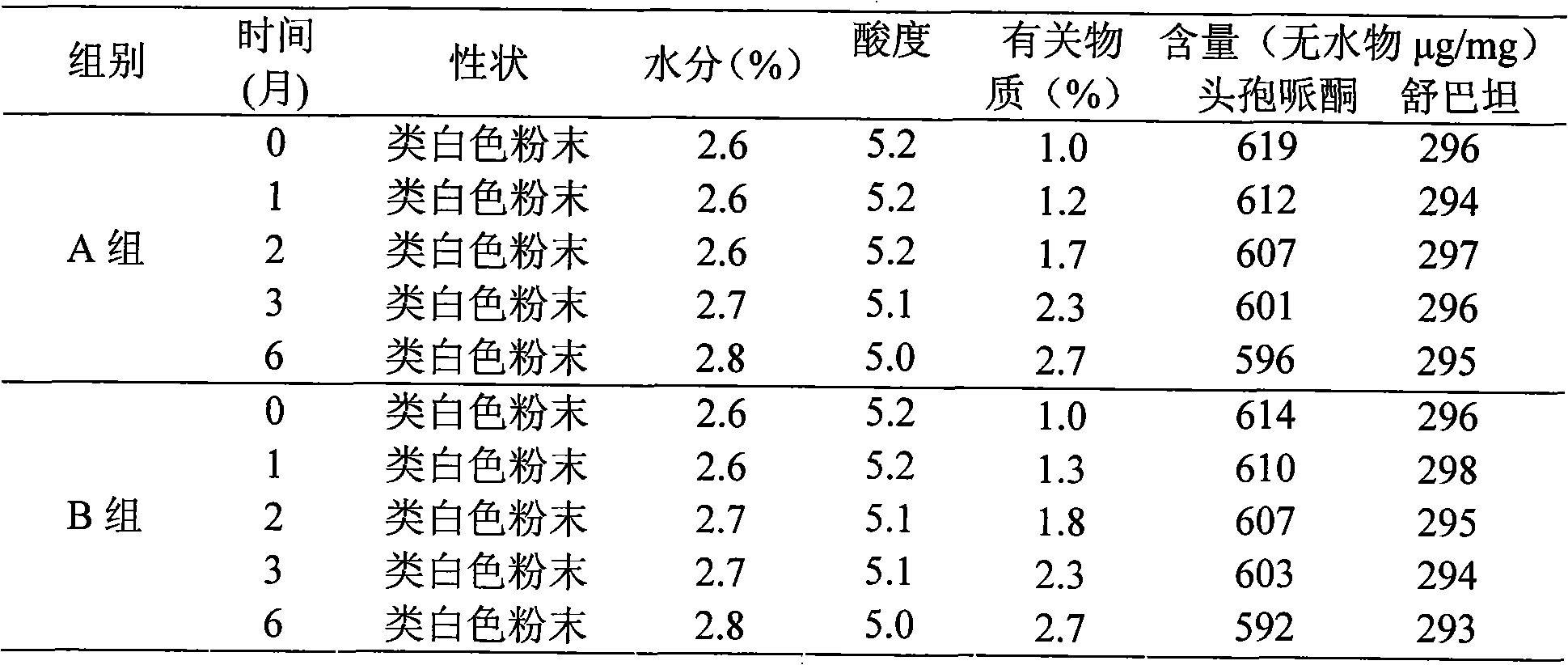
Composition of cefperazone sodium and sulbactam sodium and lysine
A technology of cefoperazone sulbactam sodium and cefoperazone sodium, which is applied in the field of the composition of cefoperazone sulbactam sodium and lysine, can solve the problems of poor stability, accumulation and aggravation of kidney damage of cefoperazone sodium, and achieve good kidney safety Sexuality, improvement of curative effect, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0083] The above-mentioned content of the present invention will be further described in detail through specific implementation in the form of examples below. However, it should not be construed that the scope of the above-mentioned subject matter of the present invention is limited to the following examples. All technologies realized based on the above contents of the present invention belong to the scope of the present invention.
[0084] Embodiment The preparation of the composition powder injection preparation of cefoperazone sulbactam sodium and lysine of the present invention
[0085] 1. Prescription
[0086] Prescription 1
[0087] Cefoperazone sodium (in C 25 h 27 N 9 o 8 S 2 Count) 1000g
[0088] Sulbactam sodium (in C 8 h 11 NO 5 S meter) 500g
[0089] Lysine 250g
[0090] Disodium hydrogen phosphate 15g
[0091] Sodium dihydrogen phosphate 94g
[0093]
[0094] A total ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com