Cefoperazone sodium novel crystal form and its preparation method
A technology of cefoperazone sodium and crystal form, which is applied in the field of preparation of three subtypes, can solve problems such as unqualified content of cefoperazone sodium, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Classification of Cefoperazone Sodium Crystalline Samples
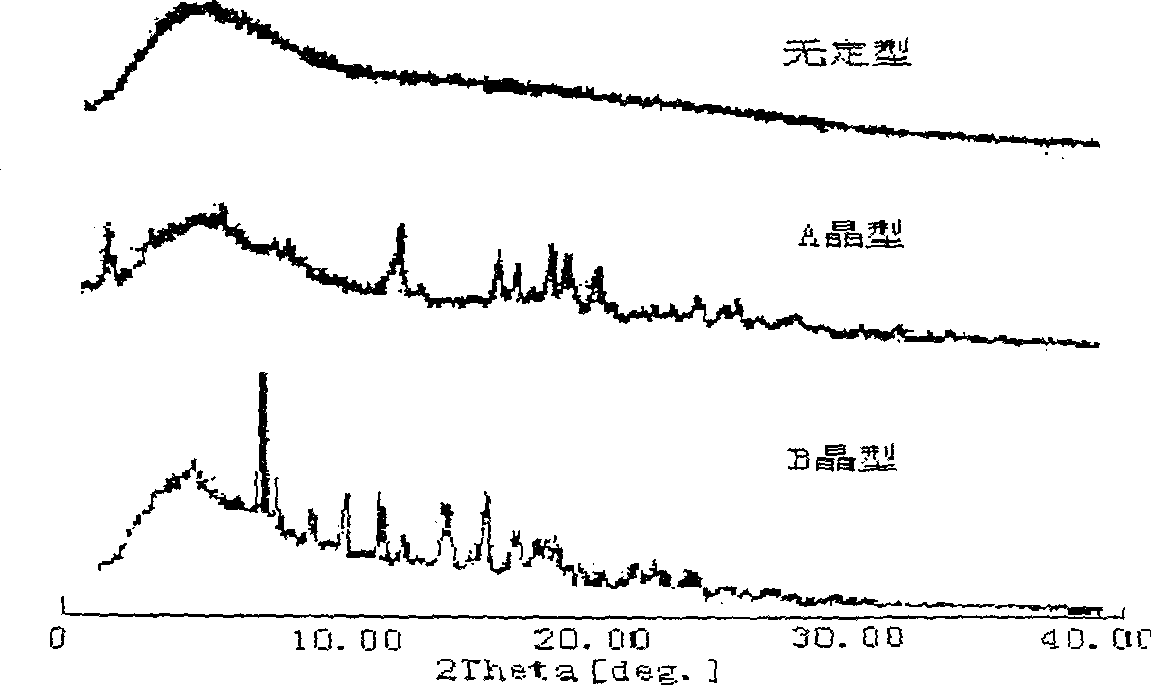
[0042] When powder X-ray diffraction analysis was performed on 112 batches of domestic cefoperazone sodium for injection, it was found that they were both crystalline samples but had two different diffraction patterns. The distribution positions and intensities of the diffraction peaks of the two were different. See figure 1 . It is suggested that the crystalline form of cefoperazone sodium may have two different crystal forms, which we call the A crystal form and the B crystal form respectively. The I / I 0 , 2θ, and d / n values (d / n values are calculated by Bragg equation 2d sinθ=nλ) are listed in Table 1 respectively. It can be seen that the d / n values of crystal form A and crystal form B are significantly different, further indicating that there are two different crystal forms of crystalline cefoperazone sodium.
[0043] Table 1 Powder X-ray diffraction data of two different crystal forms of...
Embodiment 2
[0045] Embodiment 2 Comparison of the stability of crystal form A and crystal form B
[0046] 2.1. Lattice stability
[0047] 2.1.1 High temperature powder X-ray diffraction experiment
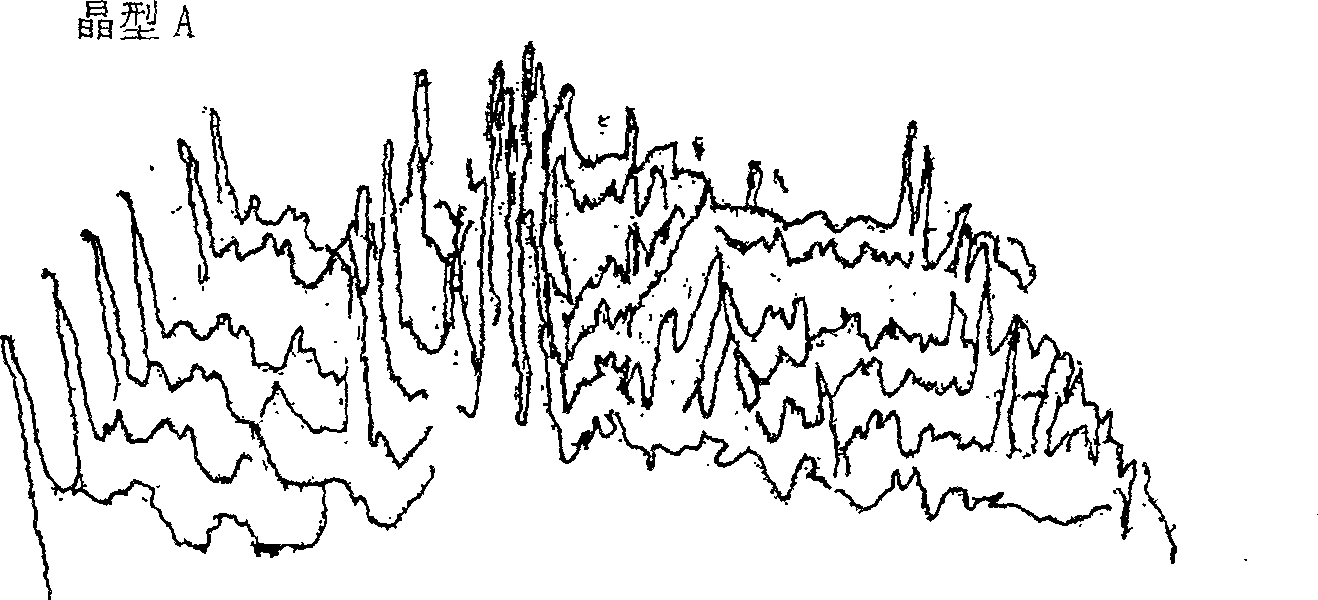
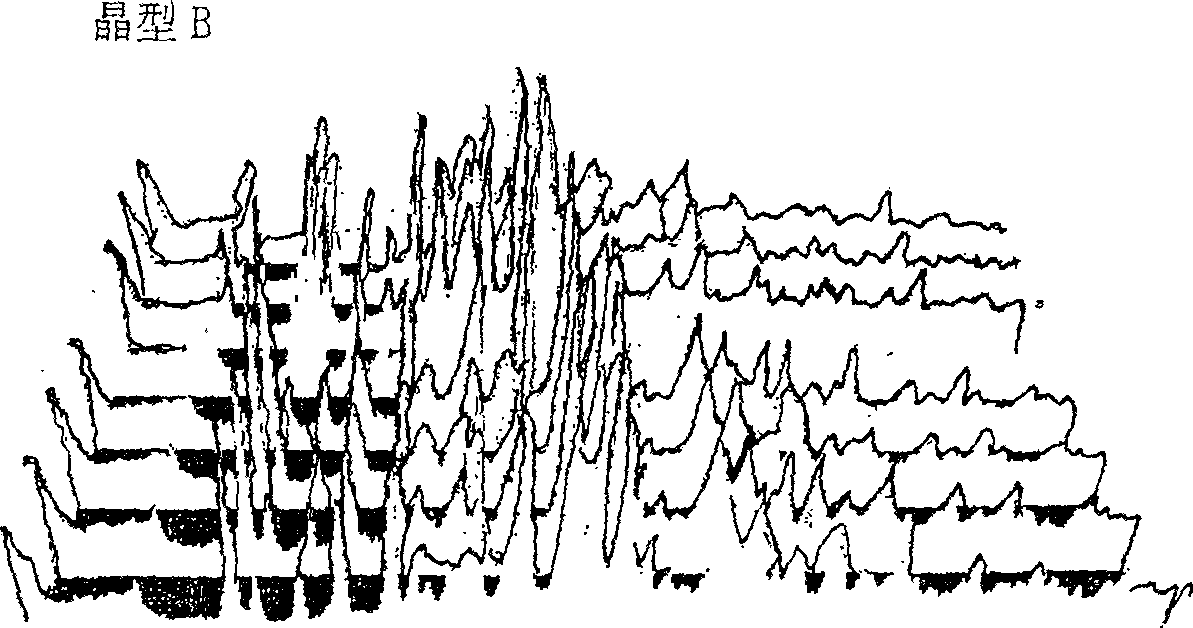
[0048] High-temperature powder X-ray diffraction experiments were carried out on the two crystal forms of cefoperazone sodium A and B, and it was found that when the crystal form A was heated to 120°C, the peak intensity of the diffraction peak began to weaken; The single peak is then divided into two diffraction peaks with lower intensity; when the temperature reaches 140°C, the positions and intensities of each diffraction peak do not change much in the diffraction pattern, indicating that the crystal lattice has not changed much, and the thermal stability is relatively high. it is good. The high-temperature powder X-ray diffraction patterns of cefoperazone sodium A and B crystal forms are shown in figure 2 and image 3 .
[0049] 2.1.2 Thermal analysis experiment
[0050] The melting...
Embodiment 3
[0056] Example 3 Cluster Analysis of Cefoperazone Sodium Form A Samples
[0057] A total of 24 batches of cefoperazone sodium crystal form A samples obtained by different production processes were selected for powder X-ray diffraction experiments, and the spectra were analyzed by random RISM.Qualitative Analysis software to obtain the average diffraction peak position (2θ) of 10 strong peaks and the spectra of each sample The corresponding peak intensity (I), the results are shown in Table 2-1, and the relative peak intensity (I / I 0 ), the results are shown in Table 2-2. Using the systematic clustering method provided by SPSS software to classify the A crystal form samples, the A crystal form cefoperazone sodium samples can be further divided into 5 subtypes: the samples numbered 13, 14, 15, 6 and 7 are the first subtype; Samples numbered 4, 17, 12, 19, 11, 8, 10, 1, 2, 3, 16, and 24 were subtype II; samples numbered 5, 20, and 18 were subtype III; samples numbered The sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com