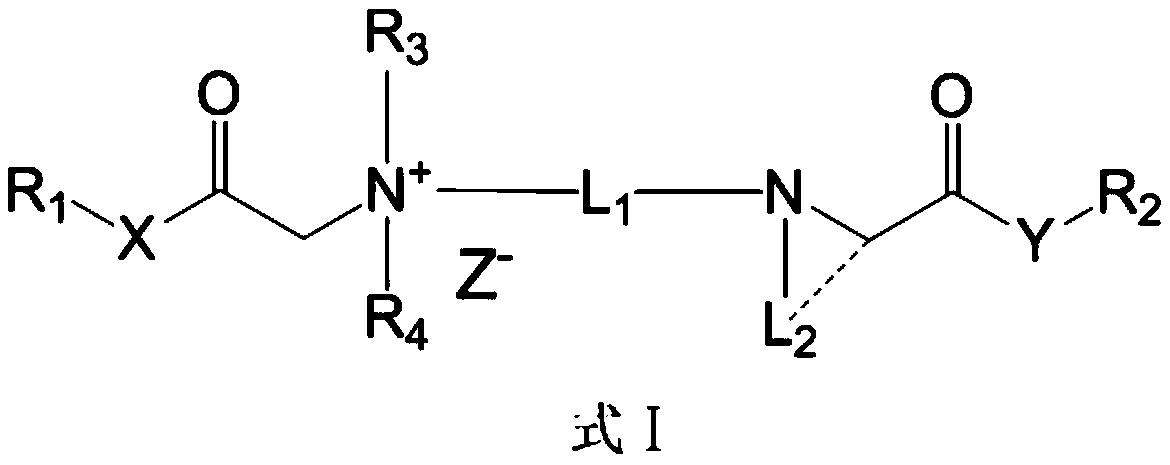
Cationic compound and preparation method and application thereof
A compound and solvate technology, applied in the field of cationic compounds and their preparation and use, can solve the problems of inability to meet clinical needs, no selective local anesthesia effect, poor local anesthesia selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1, the preparation of compound of the present invention
[0104]
[0105] Lidocaine 1a (10.0g, 42.67mmol, CAS: 137-58-6) was dissolved in 15mL of 1,,3-dibromopropane, heated to 75°C for 40h, monitored by TLC (DCM:MeOH=10:1, R f = 0.3). Add an appropriate amount of ethyl acetate to form a viscous syrupy substance, pour off the supernatant, and 15g of the remaining crude product is dissolved in 30mL of methanol and then mixed with silica gel. After dry loading, the sample is purified by silica gel column chromatography. Eluent: DCM:MeOH=10:1, the eluate was collected and concentrated to obtain 7.0 g of crude product. Ethyl acetate and dichloromethane were recrystallized to obtain 6.6 g of off-white solid powder (intermediate 1b), yield: 35.5%, which was used for the next reaction.
[0106] Intermediate 1b (1.00g, 2.806mmol), N-(2,6-dimethylphenyl)-2-piperidinecarboxamide (0.717mg, 3.087mmol, CAS: 15883-20-2 ) was dissolved in 10 mL of ethanol, DIPEA (1.0...
Embodiment 2
[0107] Embodiment 2, the preparation of compound of the present invention
[0108]
[0109] Starting material 2a (10.0g, 37.83mmol) was dissolved in 20mL of 1,4-dibromobutane, heated to 75°C for 24h, monitored by TLC (DCM:MeOH=10:1, R f = 0.3). An appropriate amount of ethyl acetate was added, and the reaction solution solidified to produce a white solid. The crude product, 16.0 g of the white solid, was filtered out and purified by silica gel column chromatography. Eluent: DCM:MeOH=20:1, the eluate was collected and concentrated to obtain 5.9 g of white solid (intermediate 2b), yield: 32.5%, which was used for the next reaction.
[0110] Intermediate 2b (1.0g, 2.08mmol) and N-(2,6-dimethylphenyl)-2-piperidinecarboxamide (0.62g, 2.67mmol, CAS: 15883-20-2) prepared above Dissolve in 15 mL of ethanol, add DIPEA (0.57 g, 0.77 mL, 4.44 mol), and react at 30°C for 10 days. The solvent was evaporated to dryness, and the crude product was purified by silica gel column chromatog...
Embodiment 3
[0111] Embodiment 3, the preparation of compound of the present invention
[0112]
[0113] Starting material 3a (2.0g, 7.93mmol) was dissolved in 4mL of 1,5-dibromopentane, heated to 70°C for 24h, monitored by TLC (DCM:MeOH=20:1, R f = 0.3). An appropriate amount of ethyl acetate was added, and the reaction solution solidified to produce a white solid. The crude product, 14.0 g of the white solid, was filtered out and purified by silica gel column chromatography. Eluent: DCM:MeOH=20:1, the eluate was collected and concentrated to obtain 1.8 g of white powdery solid (intermediate 3b), yield: 47.1%, which was used for the next reaction.
[0114] Intermediate 3b (1.8g, 3.73mmol) and N-(2,6-dimethylphenyl)-2-piperidinecarboxamide (1.08g, 4.65mmol, CAS: 15883-20-2) prepared above Dissolve in 30 mL of ethanol and 5 mL of methanol mixed solvent, add DIPEA (1.00 g, 1.35 mL, 7.75 mmol), and react at 30°C for 18 days. After the reaction was complete, it was purified by silica gel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com