Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
217results about How to "Fast onset" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chinese medicinal preparation for cardio-cerebral blood vessel diseases and its making method
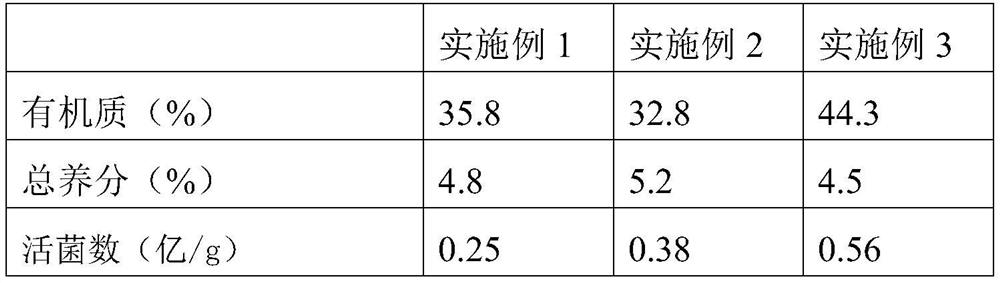
ActiveCN1679832ASimple preparation processImprove active ingredientsUnknown materialsSolution deliveryDiseaseCurative effect
A Chinese medicine in the form of tablet, capsule, particle, or oral liquid for treating cardiovascular and cerebrovascular diseases is prepared from 16 Chinese-medicinal materials including Chuan-xiong rhizome, Chinese angelica root, red peony root, astragalus root, etc. Its preparing process is also disclosed.
Owner:SHAANXI BUCHANG PHARMA
Effervescent tablet containing cefixime and its preparing method
InactiveCN100417383CEasy to storeEasy to carryAntibacterial agentsOrganic active ingredientsEffervescent tabletPharmaceutical formulation
The present invention relates to an effervescent tablet containing cefixime and its preparation method. It contains 25-400 mg of cefixime and pharmaceutically-acceptable acid-dbase pair. Besides, the pharmaceutically-acceptable filling agent, adhesive, disintegrating agent, lubricating agent, sweetener and corrective also can be added according to the requirements.
Owner:CHINA PHARM UNIV
Extracting purified ginsenoside from leaves of Panax quinquefolium and ginseng at the same time and the preparing method thereof
InactiveCN101032535ASimple processLow costOrganic active ingredientsSteroidsSide effectGinsenoside Rc
The present invention relates to Chinese medicine and its extracting and processing technology, and is especially the effective part extracted from American ginseng leaf and ginseng leaf and its preparation process. The extracted effective part contains six kinds of ginsenoside substances, including ginsenoside Re, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. Its preparation process includes water extracting American ginseng leaf and ginseng leaf in the weight ratio of 1 to 0.3, macroporous resin adsorption of the water extract liquid, water eluting to eliminate impurity, further eluting with two kinds of elutents, collecting the eluted liquid, and refining the total solid matter to reach purity up to 63.45 %. The extracted effective part has less side effects, and may be used widely in compound medicine preparation and functional food and for separating ginsenoside substance.
Owner:吉林人参研究院
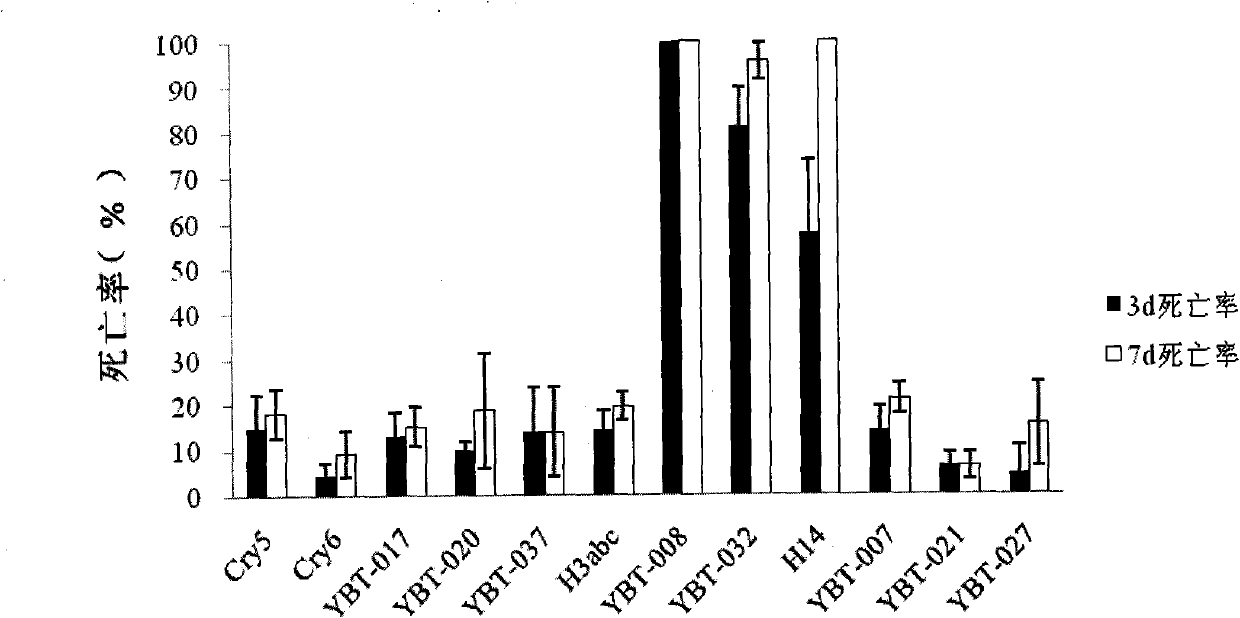
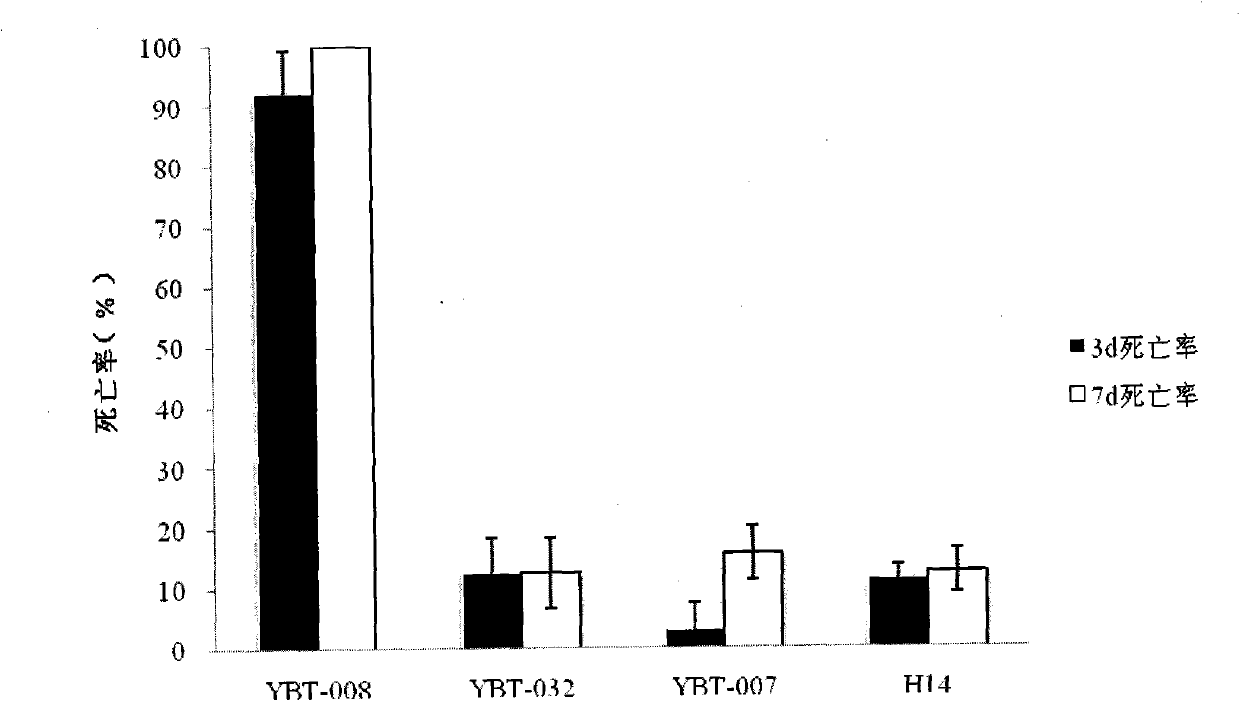
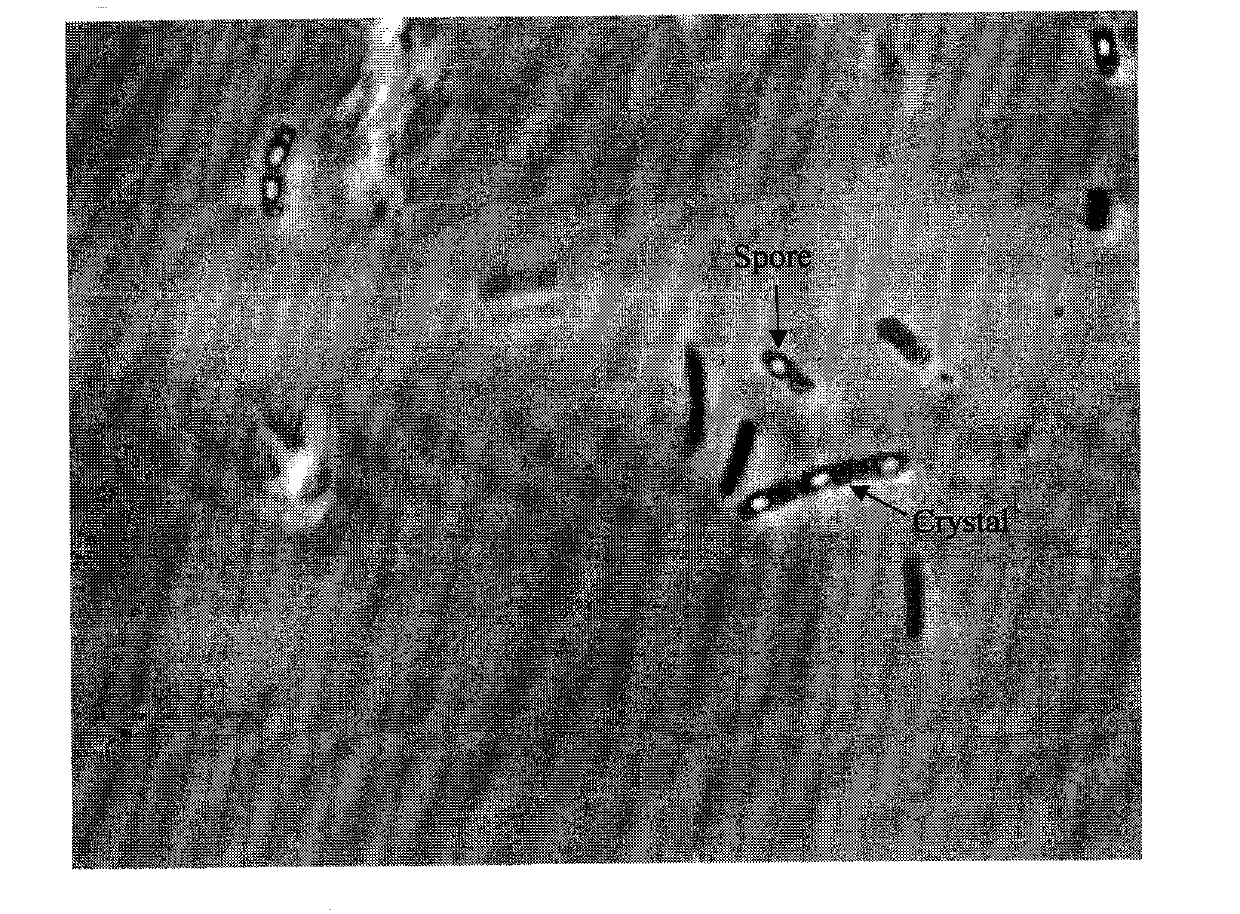
Bacillus thuringiensis YBT-008 for killing ditylenchus destructor and application thereof
The invention belongs to the technical field of microbial insecticides, and specifically relates to the screening for a bacillus thuringiensis strain with high virulence for ditylenchus destructors and the application for the prevention and treatment of ditylenchus destructors. The invention screens a bacterial strain YBT-008 with high virulence from the bacillus thuringiensis for the ditylenchus destructors. The bacterial strain is stored in China Center for Type Culture Collection, and the collection number is CCTCC NO: M2011039. The invention also includes the application effects of a microbial agent prepared by the YBT-008 on controlling the ditylenchus destructors, the types of insecticidal crystal proteins generated by the YBT-008, the verification of Cyt2Ba, Cry11Aa, Cry4Aa and Cry4Ba, and the verification of that the microbe and the microbial agent of the invention belong to a novel microbe for killing the ditylenchus destructors.
Owner:HUAZHONG AGRI UNIV
Effervescent tablet containing cefixime and its preparing method
InactiveCN1850087AEasy to storeEasy to carryAntibacterial agentsOrganic active ingredientsEffervescent tabletPharmaceutical formulation
The present invention relates to an effervescent tablet containing cefixime and its preparation method. It contains 25-400 mg of cefixime and pharmaceutically-acceptable acid-dbase pair. Besides, the pharmaceutically-acceptable filling agent, adhesive, disintegrating agent, lubricating agent, sweetener and corrective also can be added according to the requirements.
Owner:CHINA PHARM UNIV
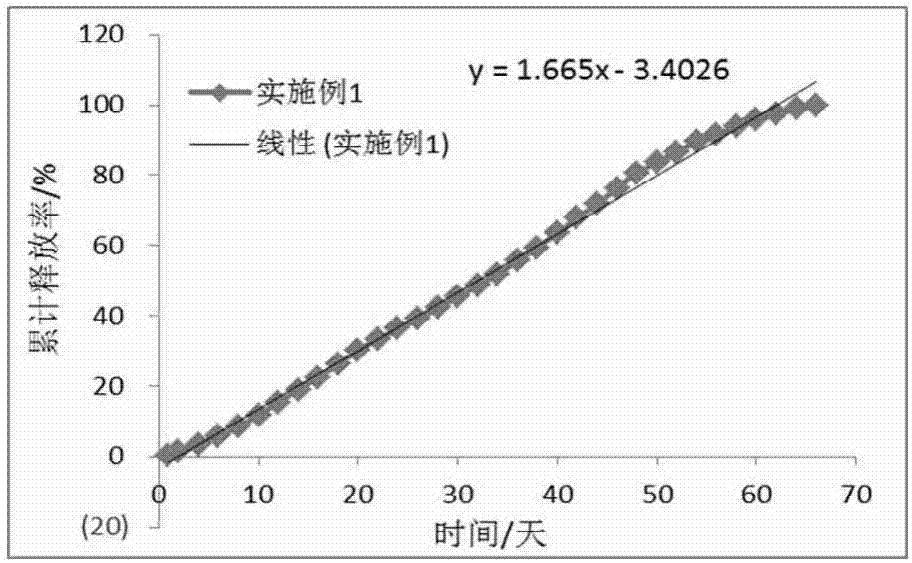
Long-acting slow-release pharmaceutical preparation and preparation method thereof
ActiveCN107213136AFast onsetEfficient preparationNervous disorderAntiviralsIntramuscular injectionWater insoluble
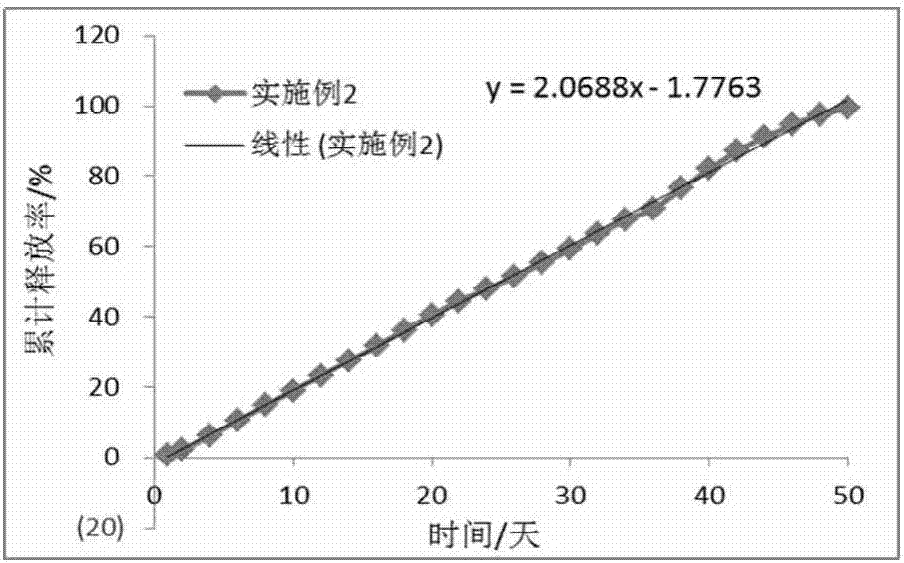
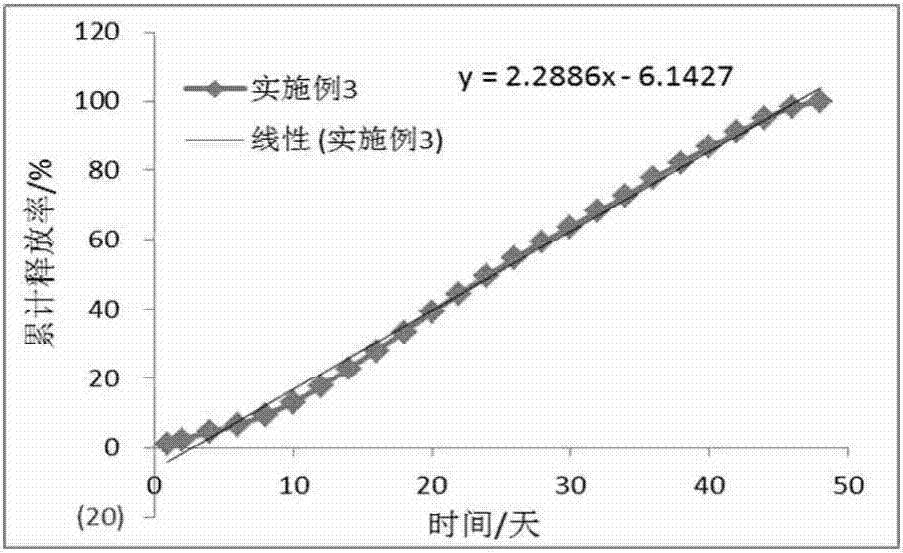
The invention discloses a long-acting slow-release pharmaceutical preparation. The pharmaceutical preparation contains the following components in percentage by weight: 25%-60% of a water-insoluble or sparingly-soluble drug and 40%-75% of a high-molecular polymer. After the pharmaceutical preparation is subjected to one time of intramuscular injection administration, the rate of a maximum plasma concentration to a minimum plasma concentration in a main release period is less than 5; the slope of a linear trend line of a cumulative release curve is less than 8 under an in-vitro simulated release condition; the daily release amount is less than 8.5%; and the simulated release condition is a buffer solution with the temperature of 37+ / -0.5 DEG C and the pH of 6.8-8.4. The prepared long-acting slow-release pharmaceutical preparation has the beneficial effects that an obvious release delay period or a burst release phenomenon are avoided after the administration, the steady state plasma concentration can be rapidly achieved, the plasma concentration with a relatively small fluctuation range can be maintained in several weeks or longer through once administration, and the pharmaceutical preparation can take effect rapidly and has good compliance with a patient.
Owner:AC PHARMA CO LTD
Zolmitriptan tongue tablet
InactiveCN101181267AAvoid first pass effectAvoid enzymatic digestionOrganic active ingredientsNervous disorderTreatment choicesSuperior vena caval
The invention provides a zolmitriptan sublingual tablet, which is characterized in that it contains the main drug zolmitriptan, a disintegrating agent and a filler. In every 1000 sublingual tablets, the content of zolmitriptan is 1 -50g, the content of disintegrating agent is 2-20g, the content of filler is 40-200g; it also contains 0-20g of corrective agent, 10-60g of binder and 0.5-5g of lubricant. The present invention utilizes the characteristics of non-keratinized sublingual mucosa, rich capillaries, and fast blood flow, aiming at the absorption of zolmitriptan ordinary tablets through the gastrointestinal tract, slow onset of action, first-pass effect, and low bioavailability. The deficiencies of advanced prior art, zolmitriptan is made into sublingual tablet, can make it absorb through sublingual mucosa, directly enter blood circulation through jugular vein and superior vena cava, take effect rapidly; Avoid oral administration The first-pass effect improves bioavailability and ensures curative effect. Moreover, water is not needed when taking the medicine, and it is placed under the tongue to contain it, so it is convenient to take and has a good taste. With the characteristics of rapid onset of action, convenient administration and high bioavailability, it can provide patients with a new treatment option and fill the gap in the market.
Owner:重庆医科大学医药研究所
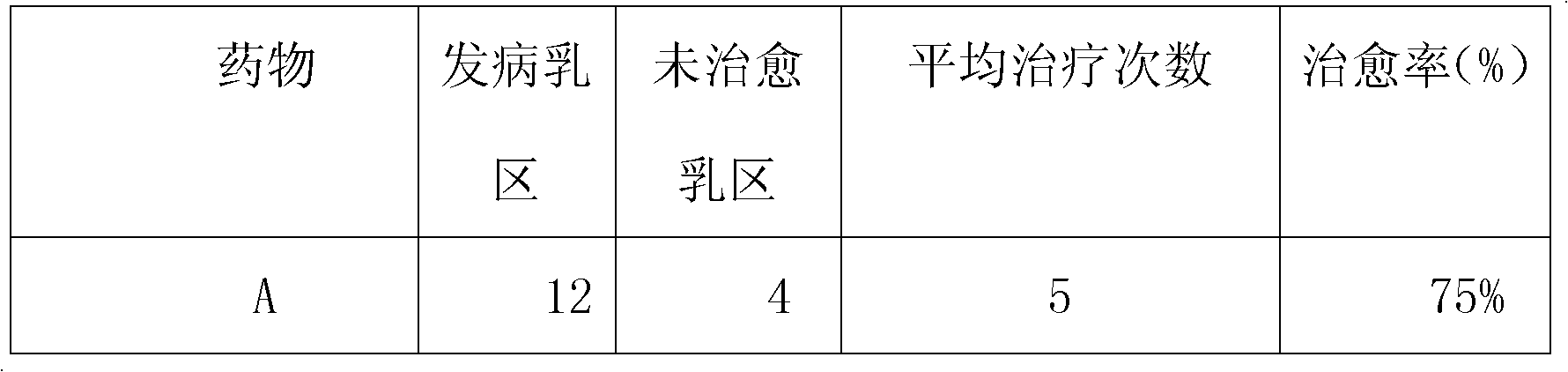
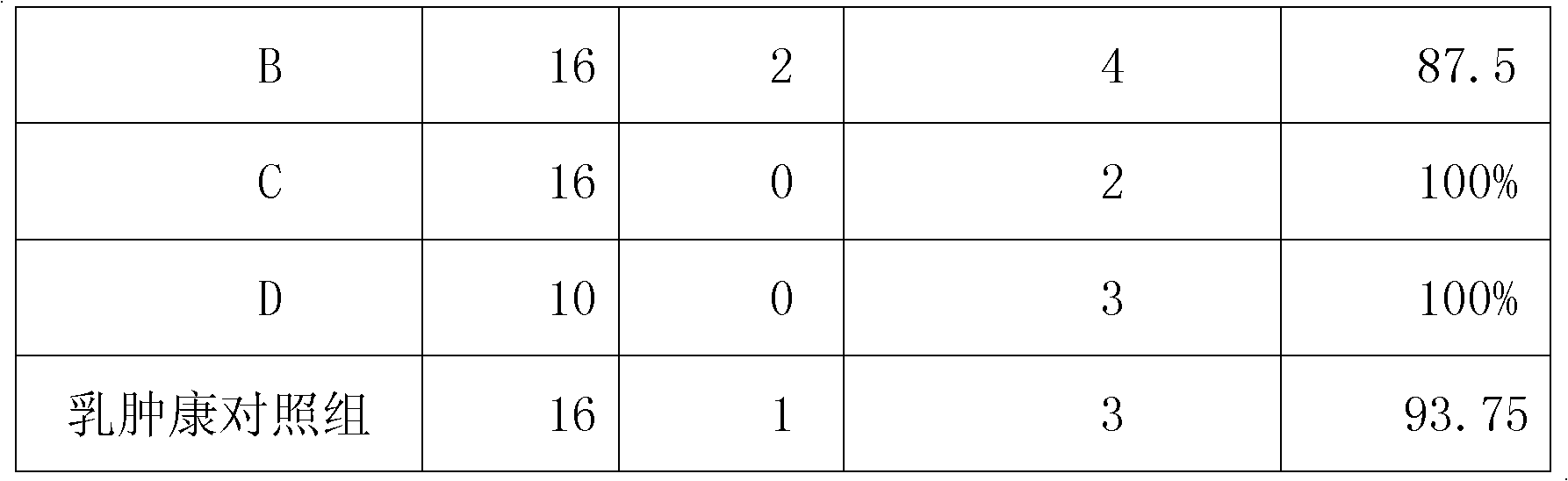
Chinese medicinal infusion solution for treating bovine mastitis and preparation method thereof
InactiveCN102119977AGood heat and detoxificationGood antibacterial and anti-inflammatoryPharmaceutical delivery mechanismSexual disorderSide effectHouttuynia
The invention relates to a Chinese medicinal infusion solution for treating bovine mastitis. The Chinese medicinal infusion solution comprises the following components in parts by weight: 1 to 10 parts of dark plum extract, 1 to 10 parts of wild jujube seed extract, 1 to 10 parts of houttuynia herb extract, 5 to 30 parts of absolute alcohol, and 10 to 50 parts of purified water. The infusion solution has high response speed, no toxic or side effect, no medicament residue, convenience in use, saving of culture cost, simplified operation and high bioavailability, and is a Chinese medicinal infusion solution for according with a safe veterinary medicament, ensuring the safety requirement of animal-derived food and treating the bovine mastitis.
Owner:TIANJIN REBATE SCI & TECH DEV
Chinese medicine liquid capsule of Folium callicarpae Nudiflorae, preparing method and quality control method
ActiveCN1814048ALess investmentShort disintegration timeAntibacterial agentsCapsule deliveryHard CapsuleSuspending Agents
The invention discloses a Nakedflower Beautyberry Leaf liquid capsule and the preparing and quality control methods thereof, characterized by that: the contents of it is prepared of the following raw materials in weight shares: dry Nakedflower Beautyberry Leaf extract powder 1, dispersant 1.2-1.6, and auxiliary suspending agent 0.04-0.07. and the preparing method: mixing the raw materials in proportion, uniformly grinding the mixture by colloid mill to make medical liquid uniform and fine, and finally making into capsules on a liquid capsule machine. It belongs to a hard capsule, and its contents are liquid, and as compared with original tablets, it has advantages of short disintegrating time, fast taking effect, high biological utilization ratio, etc.
Owner:杨文龙
Autonomous atomization wet chamber spectacles
PendingCN107080616AAvoid breedingFast onsetNon-optical adjunctsMedical devicesWater storageWater storage tank
The invention provides a pair of autonomous atomization wet chamber spectacles, which comprises a spectacles frame, water storage tanks, an elastic eyeshade, ultrasonic transduction sheets, a micro-control circuit board and a lithium battery, wherein the spectacles frame comprises a frame body, spectacles legs and lenses; atomizing nozzles are formed at the bottom of the frame body; grooves are formed in the left outer side and the right outer side of the frame body; the water storage tanks are mounted in the grooves and can be dismounted; the elastic eyeshade is mounted on one side, close to human eyes, of the frame body and can be dismounted; the micro-control circuit board is mounted in the spectacles leg on one side and controls the ultrasonic transduction sheets to operate via a program. The pair of spectacles takes effect quickly, and can quickly alleviate a xerophthalmia symptom; the water storage tank can be replaced to avoid bacterial stemming; water in the water storage tank is directly in contact with the ultrasonic transduction sheets; guide tubes are not required to further avoid the bacterial stemming.
Owner:温州视佳科技有限公司
Wet chamber glasses and control method thereof
The invention discloses wet chamber glasses and a control method thereof. The wet chamber glasses comprise a glasses frame, glasses legs, an inner frame, a microprocessor and lenses arranged on the glasses frame; a fence is arranged between the inner side of the glasses frame and the inner frame fitting the face of a wearer, and heating modules in a series connection round are distributed on the inner side of the glasses frame; at least one water storage chamber and mist passages are formed in the glasses legs, the mist passages are used for being connected with the top ends of the water storage chambers and the inner side of the fence; the microprocessor is connected with the heating modules, when the glasses are worn, the glasses frame, the lenses, the inner frame, the fence and the faceof the wearer can form a sealing cavity, and the microprocessor controls the heating modules to quickly emit heat, so that water in the water storage chambers is heated and evaporated to obtain vapor, the vapor flows to the eyes of the wearer through the mist passages. The wet chamber glasses have the advantages that the speed of taking effect is high, the dry eye symptom can be quickly relieved,and a hot compress effect is achieved.
Owner:HANGZHOU REJOIN TECH
High-efficiency mothproofing agent
A high-efficiency mothproofing agent at least contains two effective mixed matters playing the role of mothproofing under room temperature, and also contains an effective matter with a mildew-proof function. The mixture with the mothproofing function is prepared by mixing camphor, santochlor and other mothproofing agents with pyrethrin. The high-efficiency mothproofing agent also contains an additive convenient for producing a forming agent, namely micromolecule inorganic silicon substance, and can belongs to the powder type, the granulated type, the sheet type, the ball type or the massive agent type. The mothproofing agent is arranged in a bag or a box made of air-permeability paper with air permeability, non-woven fabrics and thin films. The pyrethrin is quick in taking effect, has obvious effect on killing moths, not only remedies the defects of a camphor mothproofing agent, but also greatly reduces input, and is safer and more effective. The camphor and the pyrethrin do not have the mildew-proof effect, so that a mildew-proof agent is added in the mixture of the camphor and the pyrethrin, therefore, the mothproofing agent has the effects of moth and mildew prevention.
Owner:SURYAMAS DAILY CHEM TIANJIN
Drop pills of healthy energy of wrinkled gianthyssop
InactiveCN100563635CSmall molecular weightFast dissolutionAntipyreticDigestive systemFood additiveMedicine
Owner:TIANJIN TASLY PHARMA CO LTD
Dropping pills containing ursolic acid and method for preparing the same
InactiveCN101194895ASmall molecular weightFast dissolutionOrganic active ingredientsDigestive systemDrugChemical synthesis
The invention provides a medicament which has the functions of antineoplastic, oxidation resistance, anti viral hepatitis and antiphlogistic. The invention particularly relates to a dripping pill which contains ursone and a process for preparation. The invention overcomes the shortcomings of the prior dripping pills that pure natural degrees of findings which are used by the prior dripping pills are not high, and chemosynthesis findings which are commonly used are not in food additives catalogs of some countries, and tastes of the dripping pills are worse. The invention is a pharmaceutical preparation whose natural degree is higher, safety is stronger, and side effect is lower.
Owner:TIANJIN TASLY PHARMA CO LTD
Drop pills of cucurbitacine, and preparation method
InactiveCN1872076ASmall molecular weightFast dissolutionOrganic active ingredientsDigestive systemChemical synthesisFood additive
A dripping pill of cucurbitacin for treating chronic metastatic hepatitis features high natural level and safety and low toxic by-effect. Its preparing process is also disclosed.
Owner:TIANJIN TASLY PHARMA CO LTD
Anesthesia analgesia drug composition and preparation method thereof
ActiveCN108143733AFast onsetLong-lasting analgesic and sedative effectsPowder deliveryNervous disorderCompounding drugsNarcotic
Owner:YICHANG HUMANWELL PHARMA
Oryzanol nanocrystal capsule preparation and preparation process thereof
InactiveCN105997927ADisperse fastRapid dissolutionPowder deliveryOrganic active ingredientsSolubilityClinical efficacy
The invention belongs to the technical field of medicinal preparations, and relates to a formula and a preparation process of an oryzanol nanocrystal capsule preparation. The preparation is composed of oryzanol and stabilizers, nano-suspension is prepared, then spray dispersing agents are added into the nano-suspension, spray drying is performed to obtain oryzanol nanocrystals, and a capsule is filled to obtain an oryzanol nanocrystal capsule. The nanocrystals prepared by vortex blending are uniform in grain diameter, and solubility and dissolution rate are remarkably increased. The preparation has the remarkable advantages that the solubility of the oryzanol can be increased by the nanocrystals, bioavailability of the oral preparation is improved and is less affected by the eating condition of a patient, clinical curative effects are further enhanced, and individual difference is decreased.
Owner:QILU UNIV OF TECH
Medication for treating cardiovascular diseases, and cerebrovascular disease
InactiveCN1872099BHigh degree of naturalSmall toxicityPill deliveryGinkgophyta medical ingredientsFood additiveSide effect
A medicine for treating cardiovascular and cerebrovascular diseases is disclosed. Its advantages are high natural level and safety and low toxic by-effect.
Owner:TIANJIN TASLY PHARMA CO LTD
Composition and soft capsule for alleviating cough
InactiveCN104013608AReasonable ratioIngenious designOrganic active ingredientsPharmaceutical delivery mechanismAcute bronchitisPolyethylene glycol
The invention discloses a composition and a soft capsule for alleviating cough. The composition for alleviating cough comprises the following compositions in percent by weight: 30-100% of benzonatate, 0-60% of polyethylene glycol and 0-50% of polyol. The soft capsule comprises a soft-capsule shell and a contained substance cladded by the soft-capsule gelatin shell. The contained substance comprises the following compositions: benzonatate, polyethylene glycol and polyol. The soft-capsule gelatin shell is composed of gelatin, a plasticizer, purified water and a coloring agent. The composition for alleviating cough is simple in compositions, stable, effective, and rapid in dissolving and absorption. The soft capsule containing the above composition for alleviating cough is also provided. The soft capsule is applicable to irritable cough, paroxysmal cough and the like caused by acute bronchitis, bronchitis asthma, pneumonia and lung cancer, and is applied to prevent cough when fiberoptic bronchoscopy and laryngoscopy are performed.
Owner:安士制药(中山)有限公司
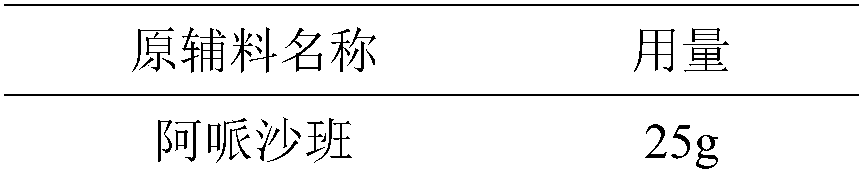
Apixaban tablet and preparation method thereof
InactiveCN108096205AIncrease dissolution rateImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineSilica gel
The invention discloses an apixaban tablet. The apixaban tablet is formed through tableting of apixaban solid dispersion and auxiliary ingredients, wherein the apixaban solid dispersion is formed by apixaban and mannitol at the weight ratio of 1 to (10-20); the auxiliary ingredients comprise microcrystalline cellulose, polyvinylpolypyrrolidone and superfine silica powder, and the weight ratio of apixaban to microcrystalline cellulose to polyvinylpolypyrrolidone to the superfine silica powder is 1 to (30-60) to (5-10) to (1-5). With the apixaban tablet provided by the invention, the defects that the in-vitro dissolution rate of apixaban is poor and the bioavailability is low are overcome. The invention further discloses a preparation method of the apixaban tablet. The method is easy to operate, the reproducibility is good, and large-batch production can be easily realized. The apixaban tablet is mainly used for resisting thrombus.
Owner:NANJING ZENKOM PHARMA
Novel medicine composition for preventing and treating chronic gastritis and relieving diseases caused by hyperacidity and preparation method thereof
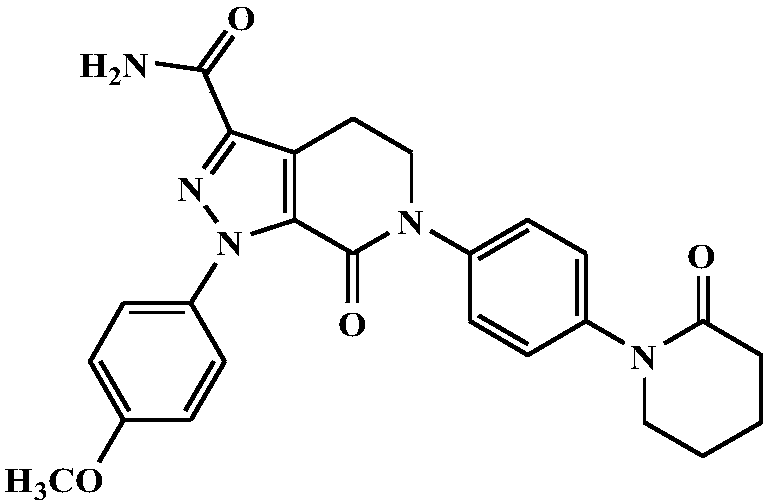
ActiveCN109771649AFast onsetRapid relief of symptomsOrganic active ingredientsPeptide/protein ingredientsCombined treatmentBiology
The invention provides a novel medicine composition for preventing and treating chronic gastritis and relieving stomachache, heartburn and sour regurgitation caused by hyperacidity. The composition isa traditional Chinese medicine and western medicine compound preparation. The compound preparation mainly contains aluminum hydroxide, radix aucklandiae, a licorice root extract and a belladonna fluid extract. The composition improves the curative effect. The invention further provides a preparation method of a medicine for traditional Chinese medicine and western medicine combined treatment of chronic gastritis and diseases caused by hyperacidity. The preparation method comprises a prescription containing traditional Chinese medicine components, a preparation process of an adhesive, preparation of a soft material, preparation of wet granules and a drying technology. The production process is optimized.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
Method for producing ultra-micro ginger powder from ginger processing by-products
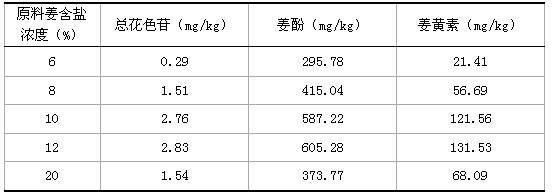
InactiveCN102599517AReduce lossIncrease profitClimate change adaptationFood preparationSlagBULK ACTIVE INGREDIENT
The invention particularly relates to a method for producing ultra-micro ginger powder from ginger processing by-products, belonging to the agricultural product processing field. The method comprises the following steps: the ginger processing by-products are washed, desalted and deacidified by clear water, and are dried in the sun or dried at the temperature of 40-60 DEG C till the moisture content of the by-products is less than 8% to obtain dry ginger slag blocks, dry ginger slag blocks are then crushed to 100+ / -20 mesh coarse ginger slag powder, and coarse ginger slag powder is crushed superfinely at the temperature of 0-5 DEG C to form 500-800-mesh fine powder, so as to obtain the ultra-micro ginger powder. The ultra-micro ginger powder prepared by the method can replace part of fresh ginger powder and is directly used for preparing ginger powder seasoning, ginger beverage, nutritional supplement, Chinese patent drug and the like. On the premise that active ingredients are not reduced, the cost of the raw material of the fresh ginger powder is greatly lowered, and the problem of environmental protection treatment for the discharge of leftovers during ginger processing is completely solved.
Owner:INST OF AGRI ENG TECH FUJIAN ACAD OF AGRI SCI
Wet tissue with effects of activating blood circulation, dispersing stasis and relieving swellings and pain
InactiveCN104352726ACtiveHas the effect of reducing swelling and relieving painAntipyreticAnalgesicsWhole bodyAdditive ingredient
The invention discloses a wet tissue with the effects of activating blood circulation, dispersing stasis and relieving swellings and pain and relates to technical field of wet tissue production. The wet tissue consists of a wet tissue matrix and a base solution attached to the matrix, wherein the base solution is prepared from the following raw materials in parts by mass: 50-65 parts of pseudo-ginseng, 30-40 parts of curculigo sinensis, 30-35 parts of dipsacus asper, 20-30 parts of murraya, 20-25 parts of pink reineckea herb, 15-20 parts of claoxylon polot merr, 15-20 parts of sappanwood, 10-15 parts of semen vaccariae, 15-25 parts of gendarussa vulgaris, 10-15 parts of garden balsam stem, 5-10 parts of notopterygium root, 10-15 parts of magnoliae officinalis, 5-10 parts of scarlet kadsura root and 5-10 parts of cortex periplocae. The wet tissue with the effects of activating blood circulation, dispersing stasis and relieving swellings and pain is prepared by using natural Chinese herbal medicines as raw materials, and the medicinal ingredients enter the body from the skin, and can perform a local therapeutic effect and achieve the purpose of treating the whole body. The wet tissue rapidly takes effect, does not have adverse reaction, and is convenient to carry and use.
Owner:铜陵麟安生物科技股份有限公司
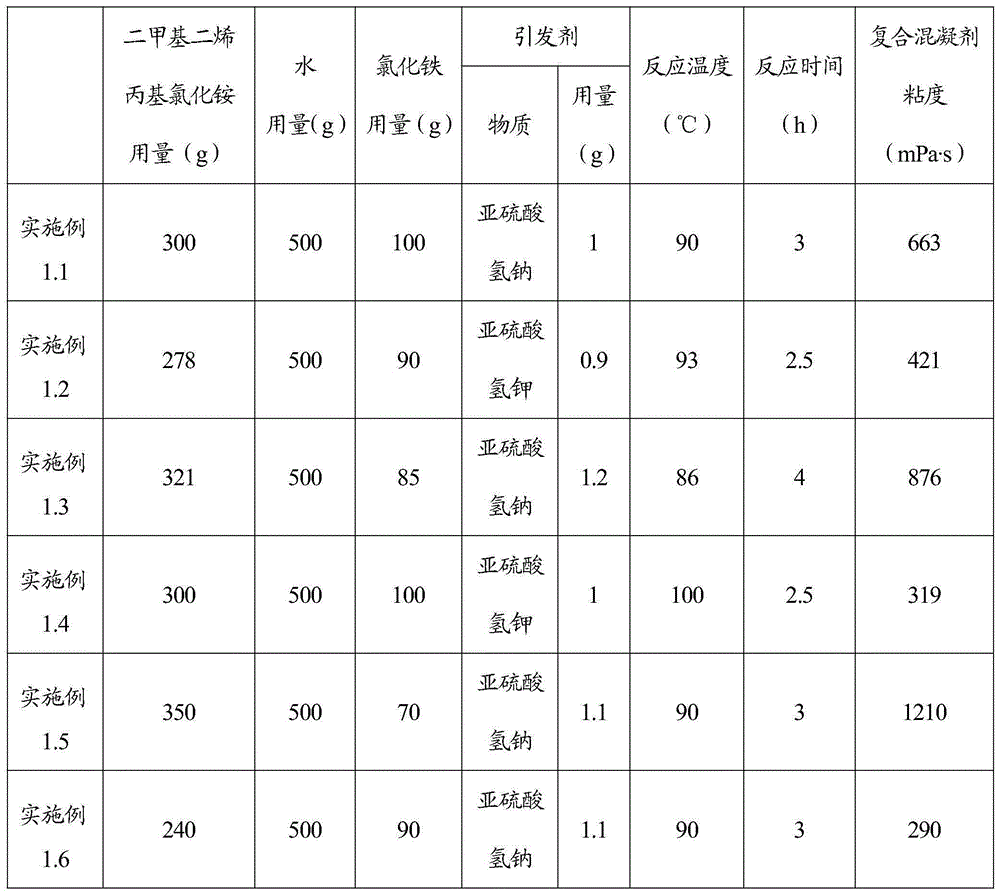
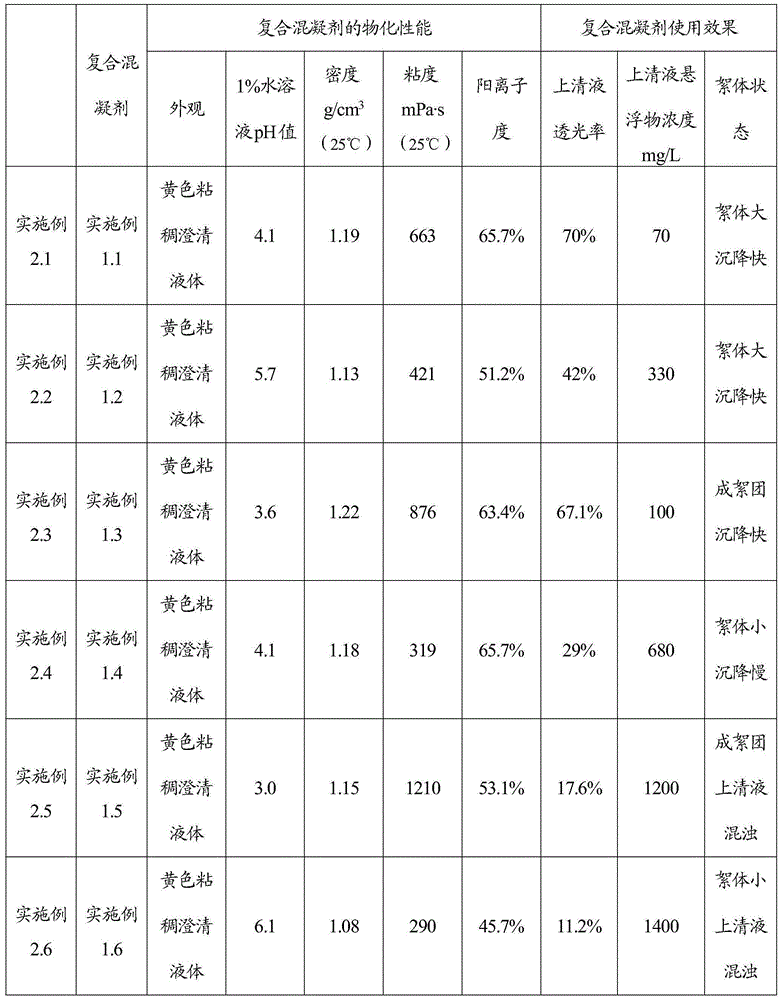
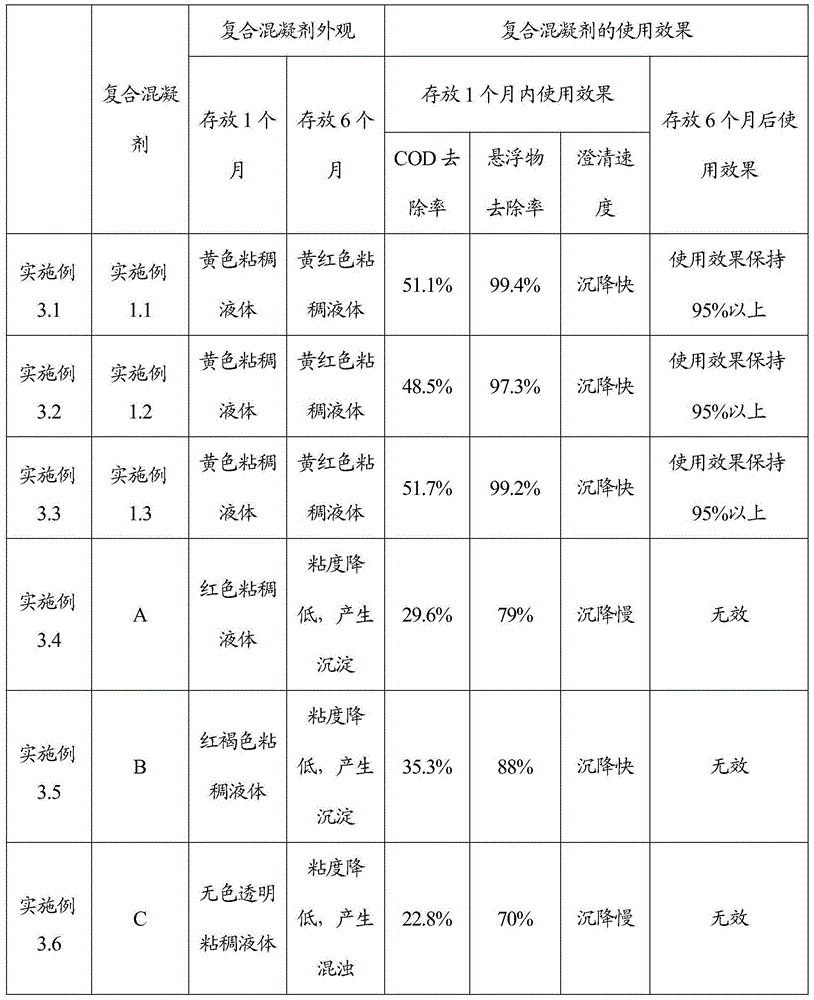
Inorganic and organic composite coagulant and preparation method and application thereof
InactiveCN105217757AGood dispersionHigh charge densityWater/sewage treatment by flocculation/precipitationWastewaterChloride
The invention relates to a preparation method of an inorganic and organic composite coagulant. Dimethyl diallyl ammonium chloride and water are mixed, ferric chloride and an initiator are sequentially added, the mixture is reacted at 85 DEG C-95 DEG C, and then the inorganic and organic composite coagulant is obtained. By means of the preparation method, the uniformity, stability and use effect of the composite coagulant can be improved, the prepared composite coagulant is not prone to layering, has good dispersion performance in use, takes effect fast and is high in sedimentation and clarification speed and good in treatment effect, and solid floc particles can be formed. The inorganic and organic composite coagulant is high in charge density and suitable for treating biological pharmacy wastewater and fermentation wastewater.
Owner:SHIJIAZHUANG LANJIANG BIOLOGICAL ENVIRONMENT PROTECTION TECH CO LTD
Preparations of artemisinin or its derivatives and amodiaquine or its derivatives and preparation method thereof
InactiveCN1709252AGood curative effectQuick and efficientHeterocyclic compound active ingredientsActive componentMedicinal chemistry
The present invention discloses a preparation made up by using artemisinin or its derivative and amodiaquine or its derivative and its preparation method. Said preparation is formed from active component and pharmaceutically-acceptable solid additive, the described active component is the mixture formed from artemisinin or its derivative and amodiaquine or its derivative. Said preparation can high-effectively and durably kill plasmodia.
Owner:桂林制药有限责任公司 +1
Drop pills for treating diabetes, and preparation method
InactiveCN1872231ASmall molecular weightFast dissolutionMetabolism disorderPill deliveryDiabetes mellitusFood additive
A dripping pill for treating diabetes features high natural level and safety and low toxic by-effect. Its preparing process is also disclosed.
Owner:TIANJIN TASLY PHARMA CO LTD
Holographic circulation repair pain-relieving external medicinal liquor for treating internal and external injury pain and preparation method thereof
InactiveCN110115751AImprove efficacyEliminate pesticide residuesNervous disorderAntipyreticCarthamusCentipede
Owner:北京德氧生健康文化传播有限公司
Multifunctional compound fermentation inoculant and preparation method and application thereof
PendingCN113444645ARapid mineralizationFast onsetBio-organic fraction processingFungiBacillus amylolyticusPaecilomyces lilacinus
The invention relates to a multifunctional compound fermentation inoculant and a preparation method and application thereof. The multifunctional compound fermentation inoculant comprises the following components in parts by weight: 5 to 15 parts of bacillus megatherium, 3 to 12 parts of bacillus licheniformis, 4 to 15 parts of bacillus amyloliquefaciens, 5 to 17 parts of bacillus pumilus, 8 to 20 parts of pseudomonas putida, 5 to 15 parts of bacillus mucilaginosus, 1 to 5 parts of aspergillus niger, 3 to 10 parts of aspergillus oryzae, 1 to 8 parts of paecilomyces lilacinus, 3 to 12 parts of trichoderma, 3 to 10 parts of streptomyces jingyangensis, 2 to 8 parts of candida utilis and 3 to 12 parts of lactic acid bacteria. Compared with the prior art, the fermentation inoculant disclosed by the invention can be mutually matched in each stage of organic waste fermentation; the fermentation inoculant is rapid to respond, has high-temperature tolerance, efficiently kills pathogenic microorganisms, can be used for preparing a bio-organic fertilizer prepared by fermenting traditional raw materials such as livestock and poultry manure and straw, can also be used for fermenting kitchen waste, municipal sludge and the like to form an organic fertilizer raw material, and has a wide application prospect.
Owner:上海又然生态科技有限公司 +1
Drop pills of 'Yixintong' and prepartion method
InactiveCN1872191ASmall molecular weightFast dissolutionPill deliveryCardiovascular disorderFood additiveChemical synthesis
A dripping pill of haw flavone for treating cardiovascular and cerebrovascular diseases features high natural level and safety and low toxic by-effect. Its preparing process is also disclosed.
Owner:TIANJIN TASLY PHARMA CO LTD
Dropping pills containing ubenimex and method for preparing the same
InactiveCN101194902ASmall molecular weightFast dissolutionOrganic active ingredientsPharmaceutical product form changeChemical synthesisFood additive
The invention provides a medicament which can enhance immune function. The invention overcomes the shortcomings of the prior dripping pills that pure natural degrees of findings which are used by the prior dripping pills are not high, and chemosynthesis findings which are commonly used are not in food additives catalogs of some countries, and tastes of the dripping pills are worse. The invention is a pharmaceutical preparation whose natural degree is higher, safety is stronger, and side effect is lower.
Owner:TIANJIN TASLY PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com