Long-acting slow-release pharmaceutical preparation and preparation method thereof
A slow-release drug and long-acting technology, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of inconvenient clinical use and poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
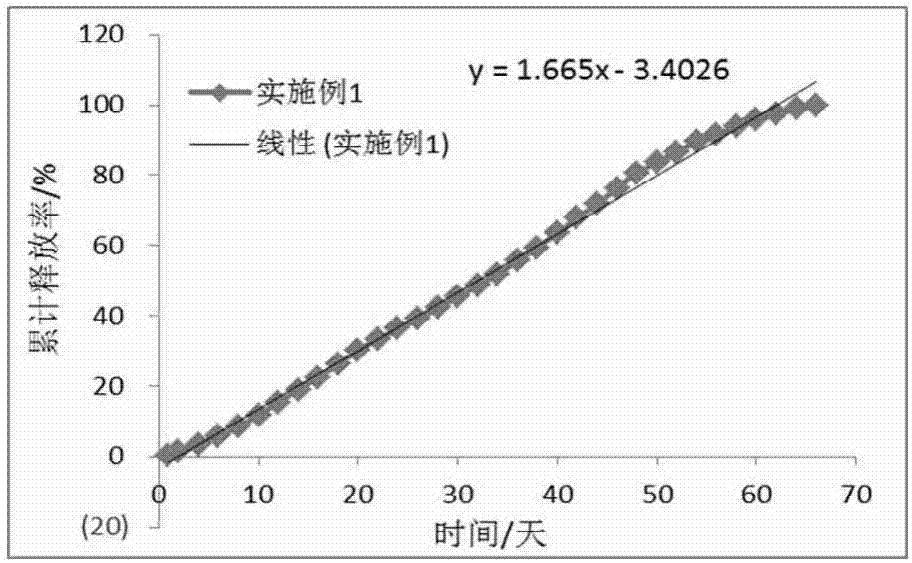
Embodiment 1
[0064] (1) Dissolve 7.5g PLGA (20000Da, 0.22dL / g, lactide / glycolide=100 / 0, COOH, COOH represents the terminal carboxyl group, the same below) in 30mL of dichloromethane, and then 2.5g of entecavir Add to the PLGA solution to dissolve to obtain the inner oil phase;
[0065] (2) Dissolving polyvinyl alcohol in purified water to a concentration of 1% and a volume of 2.4 L, then cooling to 4-8°C to obtain an external aqueous phase;
[0066] (3) Inject the internal oil phase obtained in step (1) into the external water phase of step (2) through a membrane emulsifier (average pore size is about 50 μm) to form an emulsion, then stir for 10 hours to harden the particles, collect the particles by filtration, and obtain ultra-pure Washing with water and freeze-drying for 50 hours gave long-acting slow-release entecavir microspheres with a drug loading rate of 22.59% and a geometric particle size of 28-105 μm.
[0067] The obtained microspheres were tested for their drug loading rate (H...
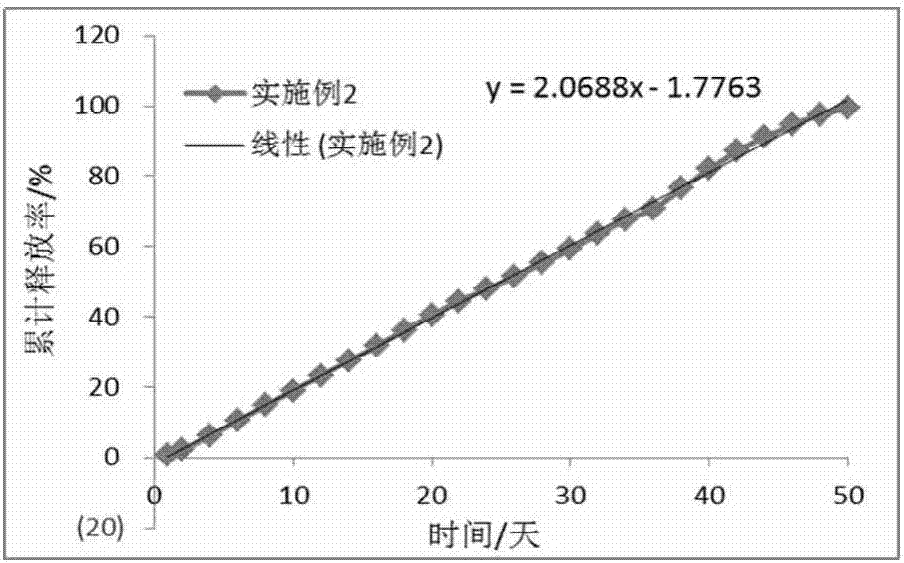
Embodiment 2
[0069] (1) Dissolve 7g of PLGA (23000Da, 0.27dL / g, 90 / 10, COOH) in 31.5mL of dichloromethane, then add 3g of anastrozole into the PLGA solution to dissolve to obtain an internal oil phase;
[0070] (2) dissolving polyvinyl alcohol in purified water to a concentration of 1% and a volume of 2.5 L, then cooling to 4-8°C to obtain an external aqueous phase;
[0071] (3) Mix the internal oil phase obtained in step (1) with the external water phase of step (2) to form an emulsion through a static mixer (L / D=30-50), then stir for 10 hours to harden the particles, collect the particles by filtration, Washing with ultrapure water, freeze-drying for 50 hours, to obtain long-acting sustained-release anastrozole microspheres with a drug loading rate of 27.11% and a geometric particle size of 32-110 μm.
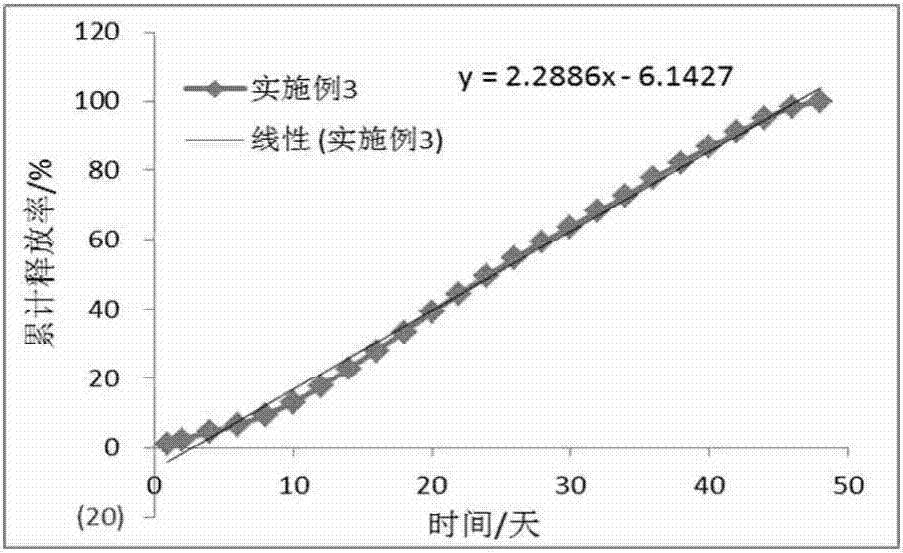
Embodiment 3
[0073] (1) Dissolve 6.5g of PLGA (22000Da, 0.25dL / g, 88 / 12, COOH) in 42mL of dichloromethane, then add 3.5g of paliperidone into the PLGA solution and dissolve to obtain an internal oil phase;
[0074] (2) Dissolving polyvinyl alcohol in purified water to a concentration of 1.5% and a volume of 3.4 L, and then cooling to 4-8°C to obtain an external aqueous phase;
[0075] (3) Mix the internal oil phase (peristaltic pump 70rpm) obtained in step (1) with the external water phase (screw pump 4m / s) obtained in step (2) to form an emulsion through a pipeline homogenizer, and then continue to stir for 10 hours to make the particles After hardening, the microparticles were collected by filtration, washed with ultrapure water, and freeze-dried for 50 hours to obtain long-acting sustained-release paliperidone microspheres with a drug loading rate of 32.08% and a geometric particle size of 25-95 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com