Application of ketamine to treatment of major depressive disorder
A depressive disorder, ketamine technology, applied in the application field of ketamine and citalopram in the treatment of major depressive disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
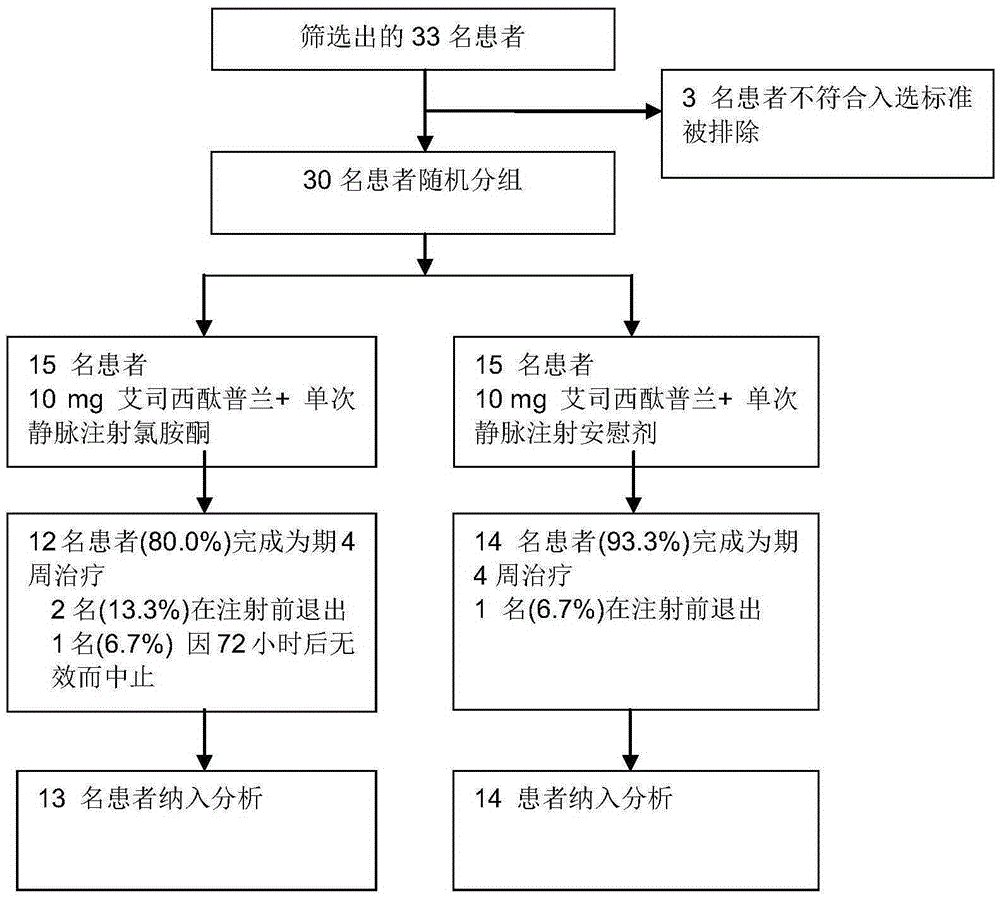
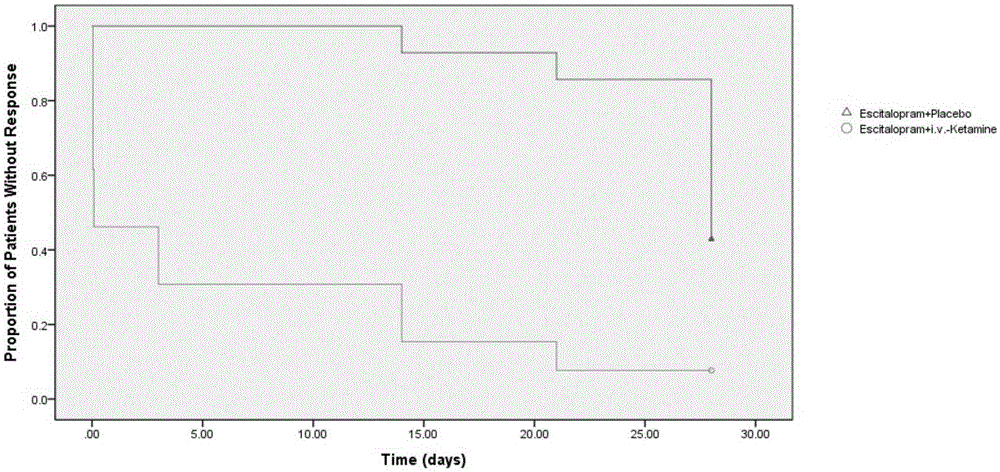
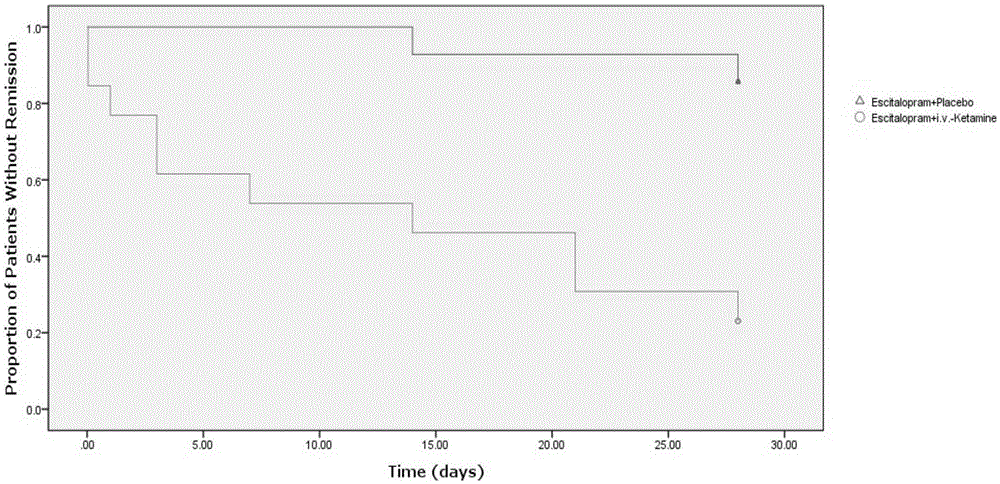
[0083] Embodiment, Ketamine Clinical Trial Efficacy Experiment
[0084] The ketamine compound used in this example is ketamine hydrochloride, the citalopram compound used is escitalopram oxalate, and the dosages used are the dosages of active drugs.
[0085] 1. Patient
[0086] From September 2013 to December 2014, a randomized, double-blind, parallel controlled trial was carried out among outpatients in the psychiatric department of Beijing Chaoyang Hospital. The trial was approved by the Human Research and Ethics Committee of Chaoyang Hospital, and each enrolled patient signed an informed consent.
[0087] To maximize the generalizability of the findings, only patients who sought psychiatric care (rather than were attracted by advertising) were included.
[0088] The inclusion criteria are: (1) 18-60 years old; (2) gender is not limited; (3) major depressive disorder without psychotic symptoms diagnosed by a psychiatrist, and confirmed with the standard scale based on DSM-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com