Xanthiphenyl ketamine or its salt and its preparing process
A technology of piperphentonamine salt and piperphentonamine salt, which is applied in the field of piperphentonamine, piperphentonamine salt and its preparation, can solve the problems of long-term effects that are yet to be seen, enhancement of myocardial contractility, intracellular Calcium overload and other problems, to achieve the effect of resisting reperfusion injury, treating heart failure, and having low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
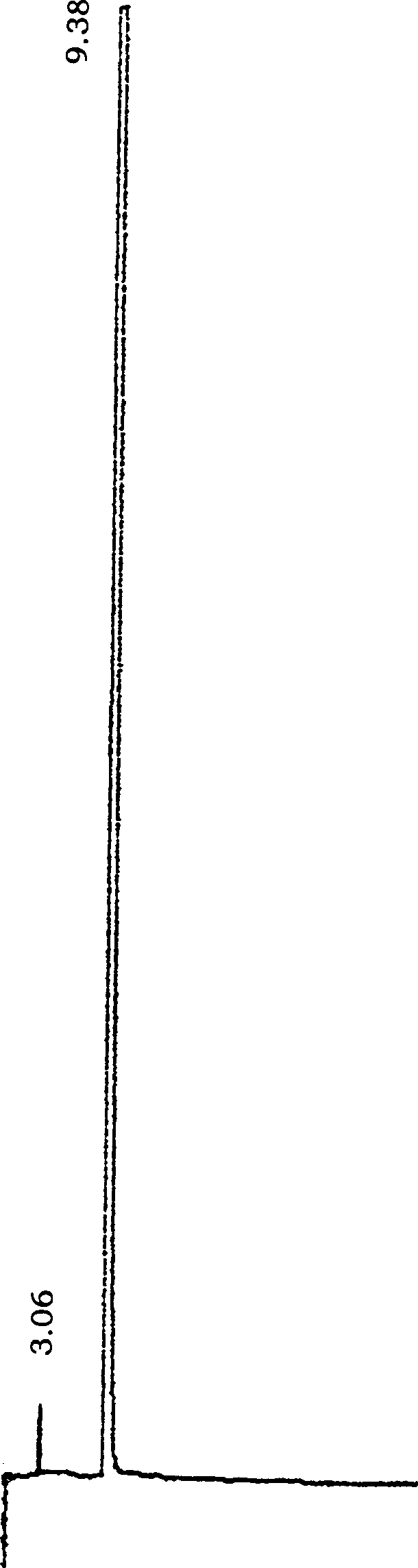
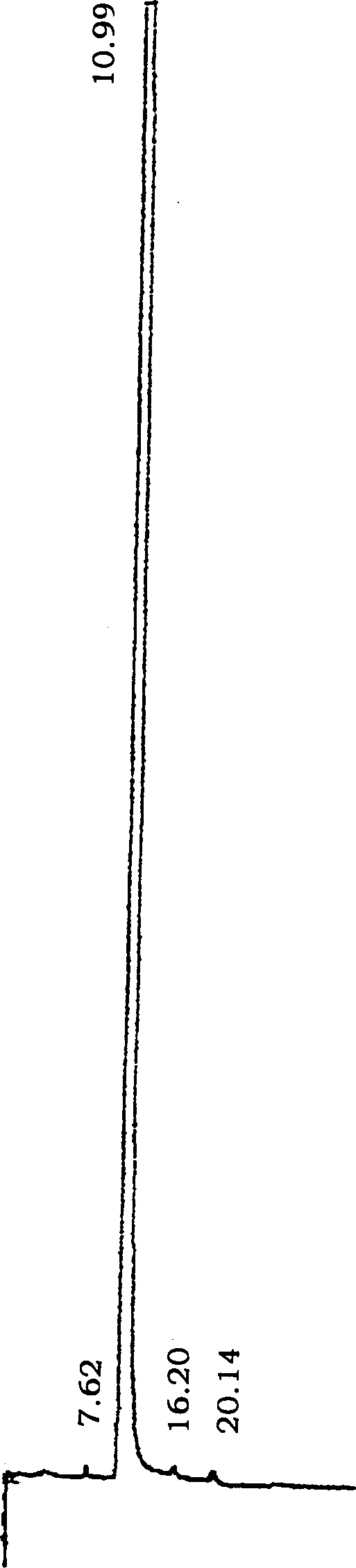
Image
Examples
Embodiment 1
[0045] Adopt the following method to synthesize N-methyl piperonyl ethylamine hydrochloride:
[0046]Put 1.09mol of piperonylethylamine (intermediate of Northeast Pharmaceutical Factory) and 1.96mol of benzaldehyde in a 1000ml round-bottomed flask, mix well, release a lot of heat, add 300ml of absolute ethanol and mix well, add zeolite, and heat with an electric heating cap , reflux reaction for 18 hours, concentrated under reduced pressure to remove ethanol, and then concentrated under reduced pressure with an oil pump to remove unreacted benzaldehyde to obtain a reddish-brown oily substance. After cooling to room temperature, add 200ml toluene and 1.09mol dimethyl sulfate. Keep the reaction at about 120°C for 30 minutes, let stand at room temperature to cool, pour off the upper toluene layer after layering, then add 300ml of 75% ethanol, and then reflux for 30 minutes to obtain a dark brown-red opaque liquid. Add 10ml of distilled water, shake well, and under stirring, use a...
Embodiment 2
[0053] Put 17.2g (0.08mol) of N-methylpiperethylamine hydrochloride and 24g (0.8mol) of paraformaldehyde in a 500ml round bottom bottle, add 200ml of absolute ethanol and 1.5ml of concentrated hydrochloric acid to make the pH about 3 -4, the temperature of the oil bath is 100°C, heat and stir until the solid is completely dissolved, then heat and stir for about 20 minutes, cool slightly, add 16.2g ((0.1mol) of p-hydroxybenzylideneacetone, reflux and stir for about 6.0 hours, the reaction Slowly separate out solid in the process, until all solidify in the reaction bottle.Detect R with TLC f =0.38 (developing agent: CH 2 Cl 2 :CH 3 OH:HCOOH=10:0.8:0.08), the reaction is basically complete. Filtered, the solid was repeatedly washed with absolute ethanol, and dried. A pale yellow solid was obtained. The solid was recrystallized with 95% ethanol to obtain a light yellow powdery solid, which was then cooled, filtered, and washed with absolute ethanol to obtain a near-white powd...
Embodiment 3
[0055] Put 25.8g (0.12mol) of N-methylpiperethylamine hydrochloride and 36g (1.2mol) of paraformaldehyde in a 500ml round bottom bottle, add 320ml of absolute ethanol and 2.0mol of concentrated hydrochloric acid to make the pH about 4 , the oil bath temperature is 90°C, heat and stir until all the solids are dissolved, then heat and stir for 10 minutes, cool slightly, add 16.2g (0.1mol) of p-hydroxybenzylidene acetone, reflux and stir for about 9.5 hours, and slowly Solids were precipitated until all solidified in the reaction bottle. Detection of R by TLC f =0.38 (developing agent: CH 2 Cl 2 :CH 3 OH:HCOOH=10:0.8:0.08), the reaction is basically complete. After filtration, the solid was repeatedly washed with absolute ethanol and dried to obtain a pale yellow solid. The solid was recrystallized with ethanol to obtain a light yellow powdery solid of piperphentonamine hydrochloride, which was then cooled, filtered, and washed with absolute ethanol to obtain a nearly white ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com