Formulations of Human Growth Hormone Comprising a Non-Naturally Encoded Amino Acid
a human growth hormone and amino acid technology, applied in growth hormones, inorganic non-active ingredients, immunological disorders, etc., can solve the problems of short in vivo half-life of proteins, undesirable side effects, decreased bioavailability and pain at injection sites, etc., to reduce biological activity, reduce formation, and be readily formulated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods for Formulations for Met-Y35pAF hGH
[0383]This example describes a formulations study that identified and evaluated suitable conditions and excipients that preserve the protein structure and activity of Met-Y35pAF hGH during storage before conjugation with PEG. Liquid formulations of hGH are frozen formulations which contain (1) 2.5 g / L sodium bicarbonate, 20 g / L glycine, 2 g / L mannitol, 2 g / L lactose, at pH 7.3; or 2) sodium citrate, 20 g / L glycine, 5 g / L mannitol, at pH 6.0. An estimated dose of 0.5-4 mg / ml of PEG-hGH is added as an active ingredient. These formulations are currently stored at −20° C. Development of a single-dose lyophilized formulation of PEGylated hGH comprising a non-naturally encoded amino acid or liquid formulation of PEGylated hGH comprising a non-naturally encoded amino acid is desired.
Methods
[0384]Several methods were implemented to characterize the physical and chemical stability of Met-Y35pAF hGH. These methods may be used in the for...
example 2
Results of Formulation Study for Met-Y35pAF hGH
Buffer pH
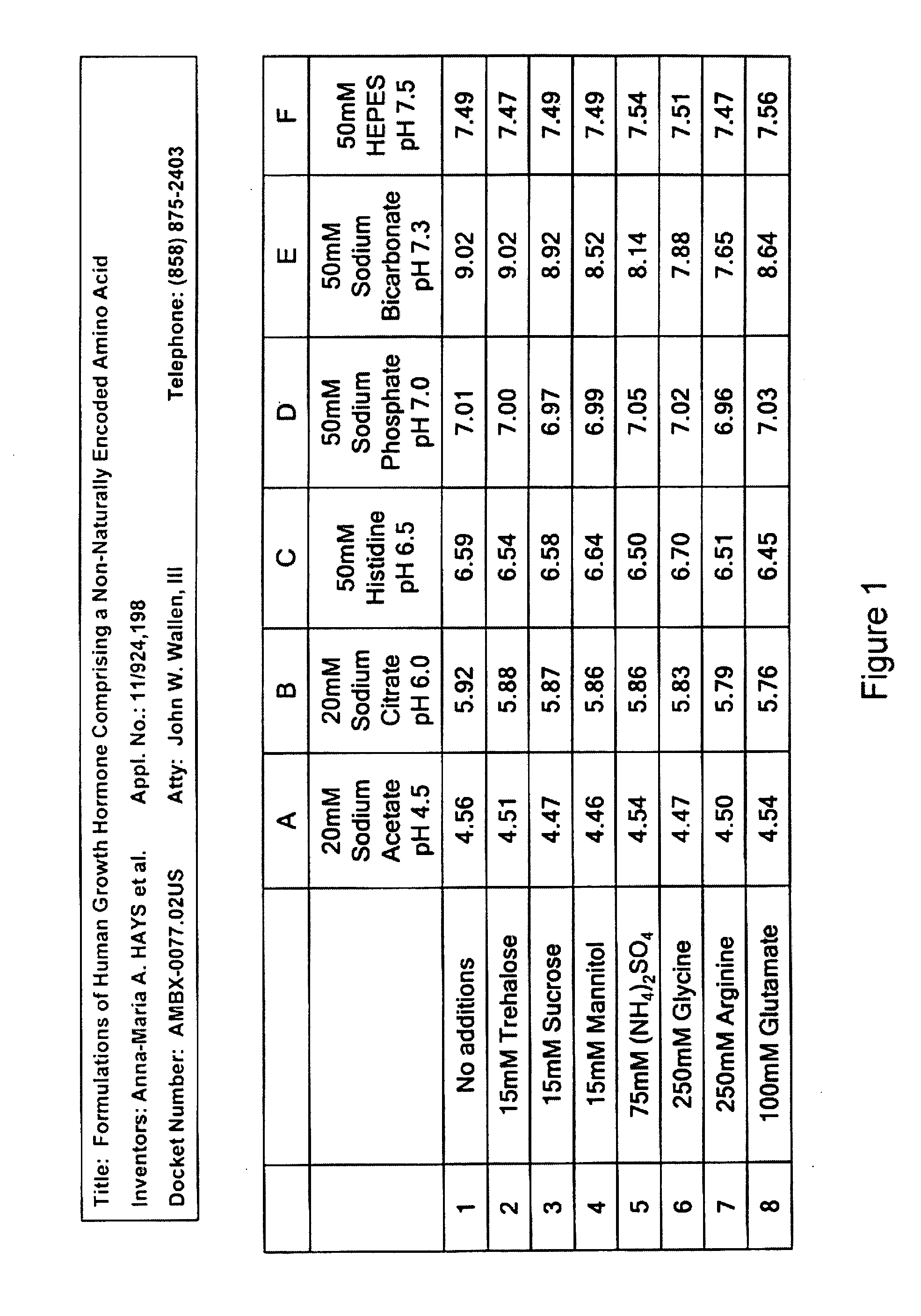
[0394]The pH of all formulation buffers was analyzed after six weeks at 4° C. It was found that the pH of all buffers chosen was stable over a six week period, with the exception of the sodium bicarbonate buffer (Group E) as shown in FIG. 1. The pH of Group E increased over time.
SDS-PAGE-R and SDS-PAGE-NR
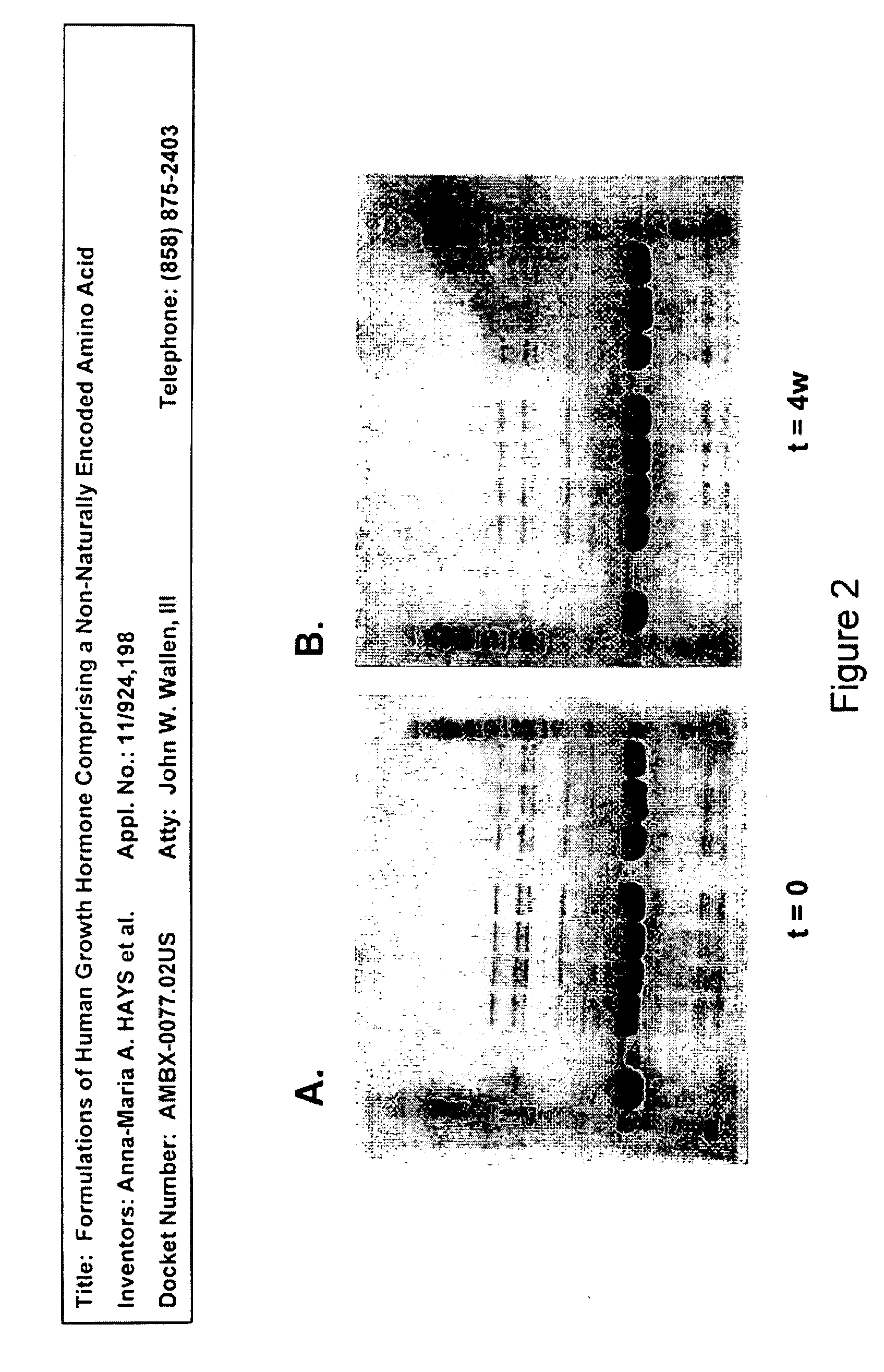
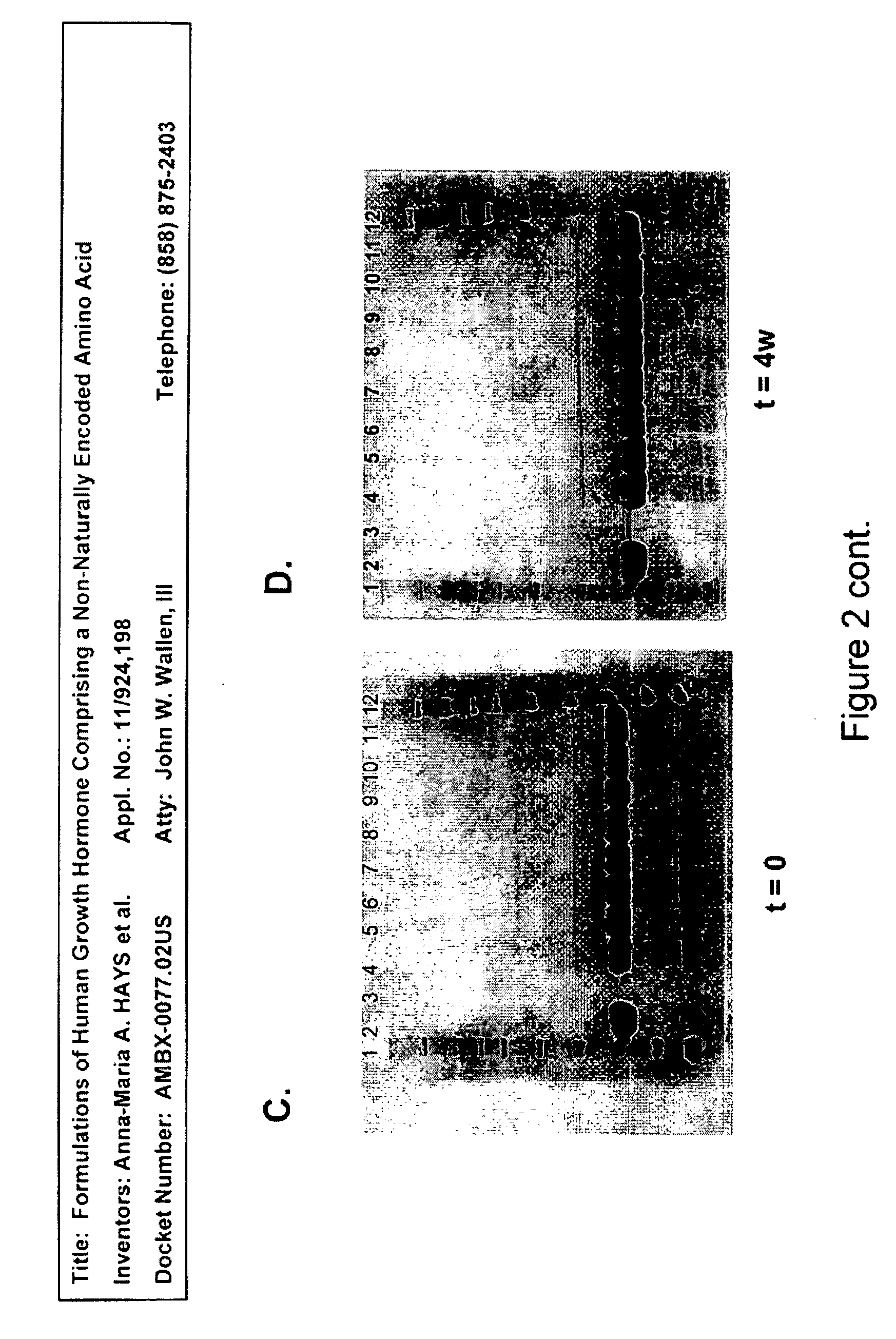
[0395]Analysis of samples with SDS-PAGE reducing and non-reducing gels showed no change either after four weeks (1 month) at 4° C. or during five freeze / thaw cycles. No dimer formation or degradation products were found by this method. Reducing gels for group A (at t=0, t=4 weeks at 4° C.) are shown as FIGS. 2C and 2D, and non-reducing gels for group B (at t=0, t=4 weeks at 4° C.) are shown as FIGS. 2A and 2B.
Differential Scanning Calorimetry (DSC)
[0396]The effects of buffer and excipient on thermal stability of MetY35pAF hGH are shown as FIGS. 3-8. FIG. 3A-F shows overlaid thermoprofiles of formulation groups A-F. FIG. 5 provi...
example 3
[0403]The stability profile of PEG-hGH is examined with key formulation parameters under accelerated stability conditions, major degradation products are identified, and stability indicating assays are confirmed. The main objective of single-dose formulation development is to optimize the formulation for sufficient storage stability minimally at refrigerated temperature and may have sufficient storage stability when stored at ambient temperature. In addition to the lyophilized formulation development, additional studies are investigating the sensitivity to light exposure and agitation, development of the RP-HPLC method distinguishing the PEGylated hGH from the non-PEGylated form, structural analysis, and other studies. Successful lyophilized formulations normally show the following attributes: stability in solution for handling during formulation and fill-finish, good stability during freeze-drying, good storage stability, native structure in the dried state (if relevant), no obviou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com