Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Stability indicating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
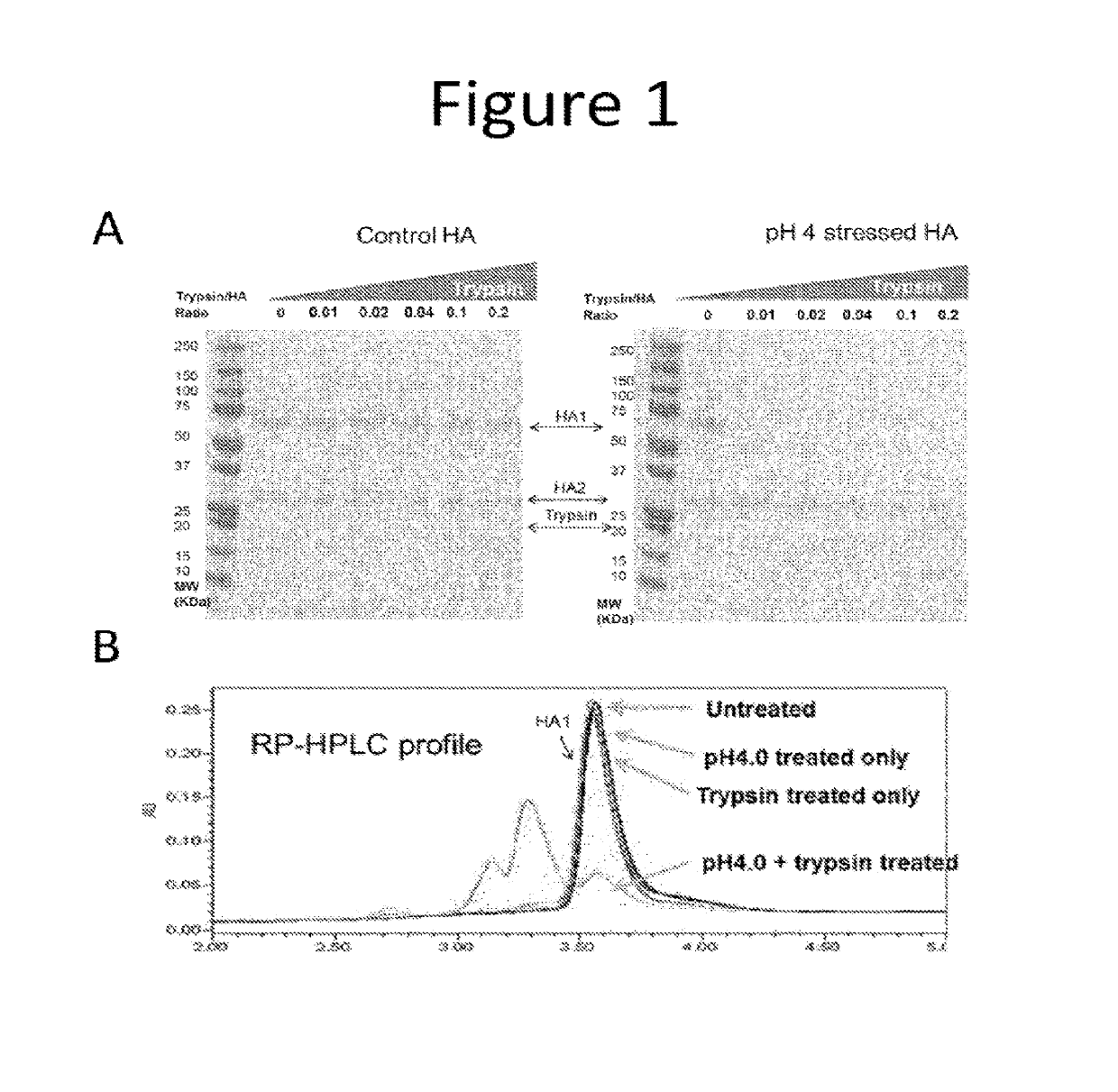
Stability indicating methods are defined by the US Food and Drug Administration as quantitative analytical methods that “are based on the characteristic structural, chemical or biological properties of each active ingredient of a drug product” and “will distinguish each active ingredient from its degradation products so that the active ingredient ...
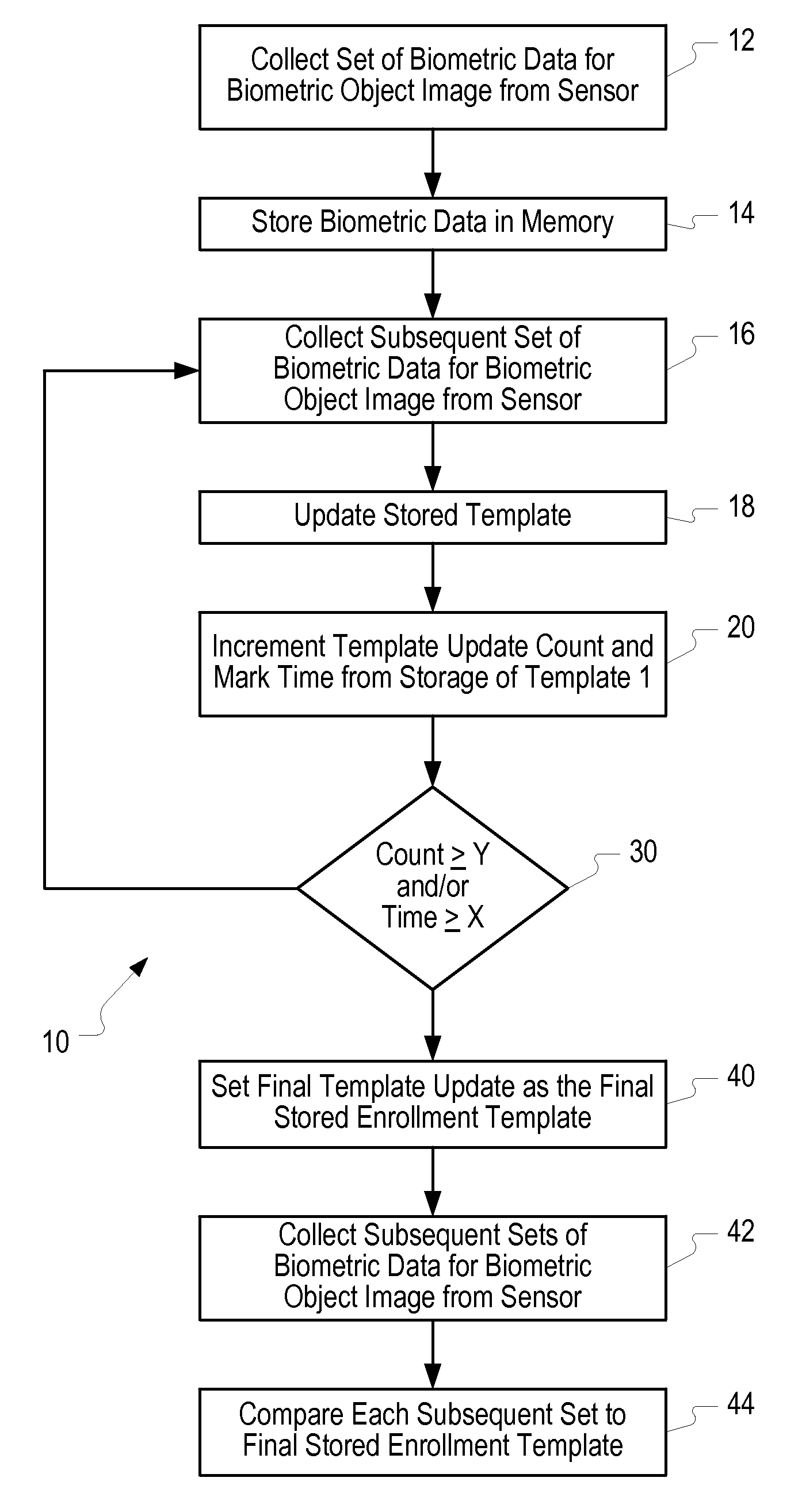
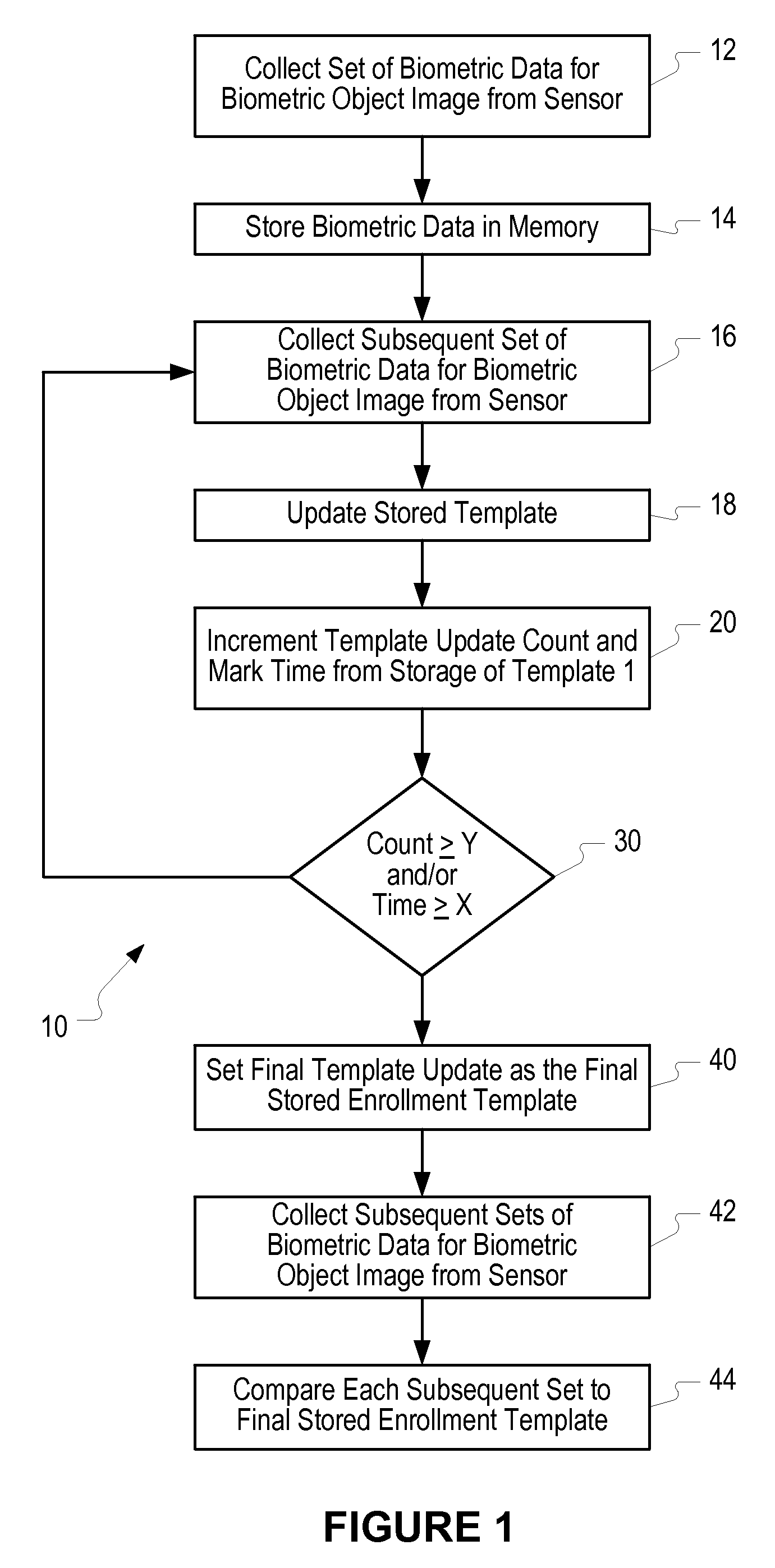
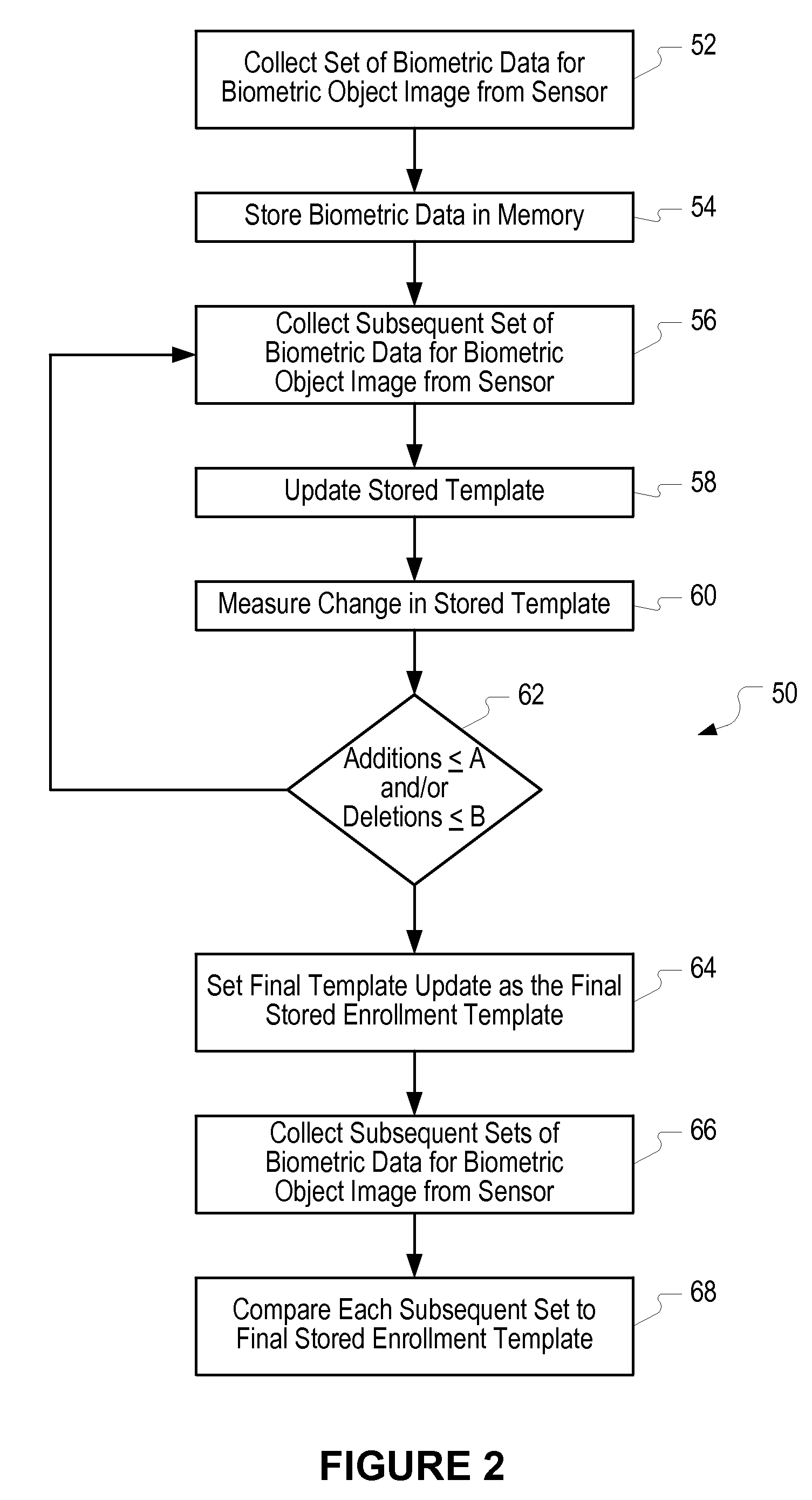
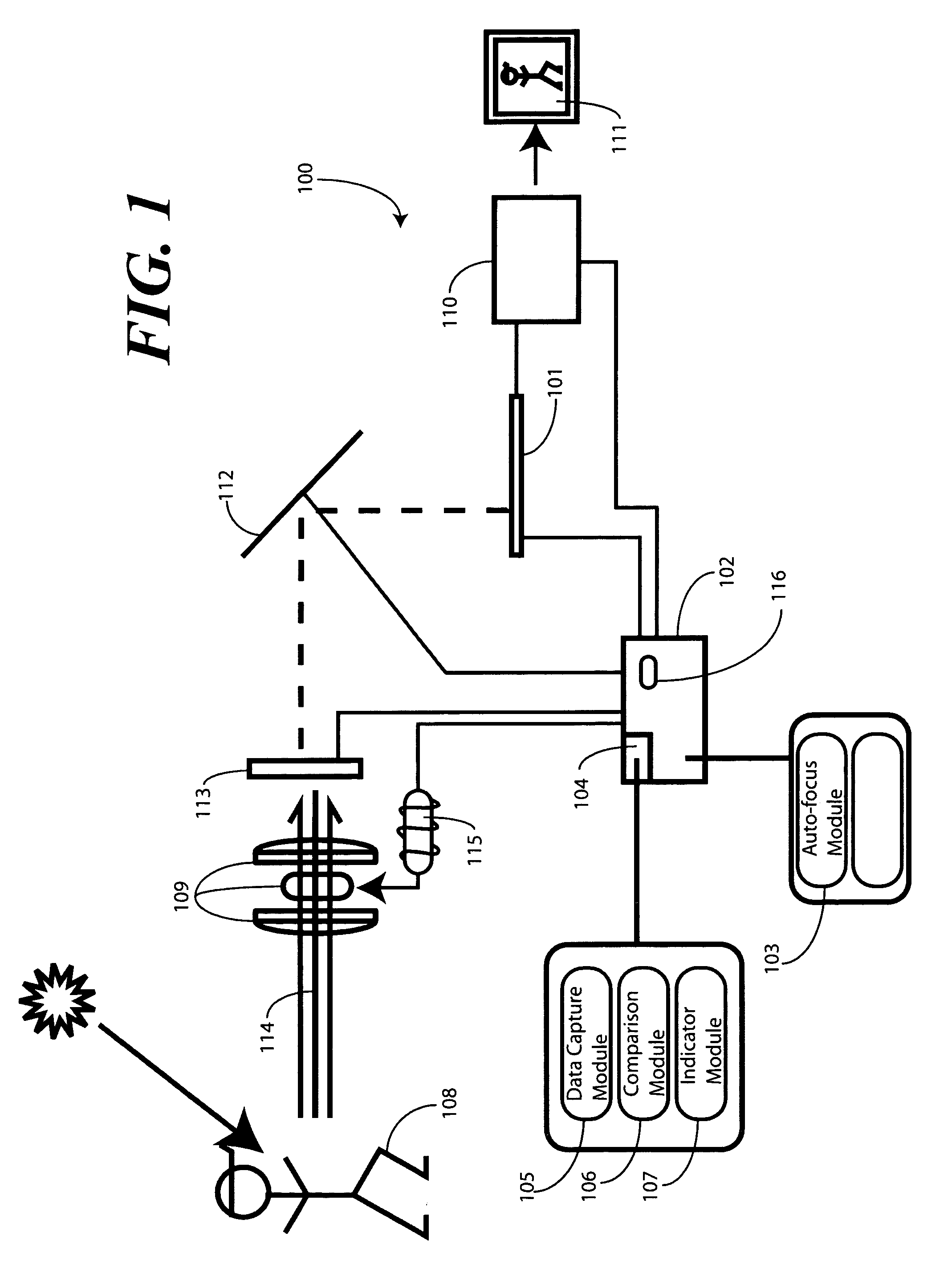
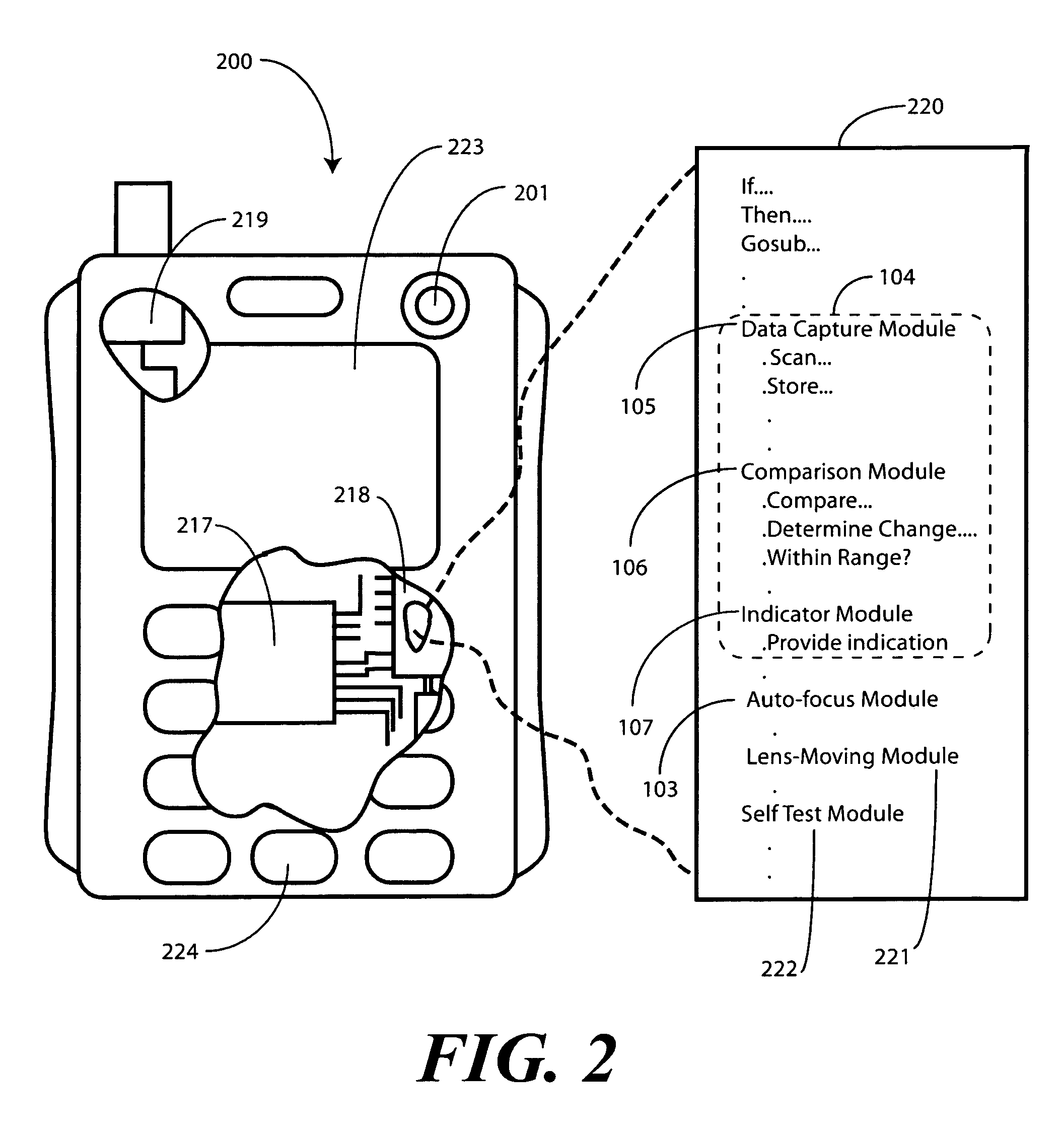
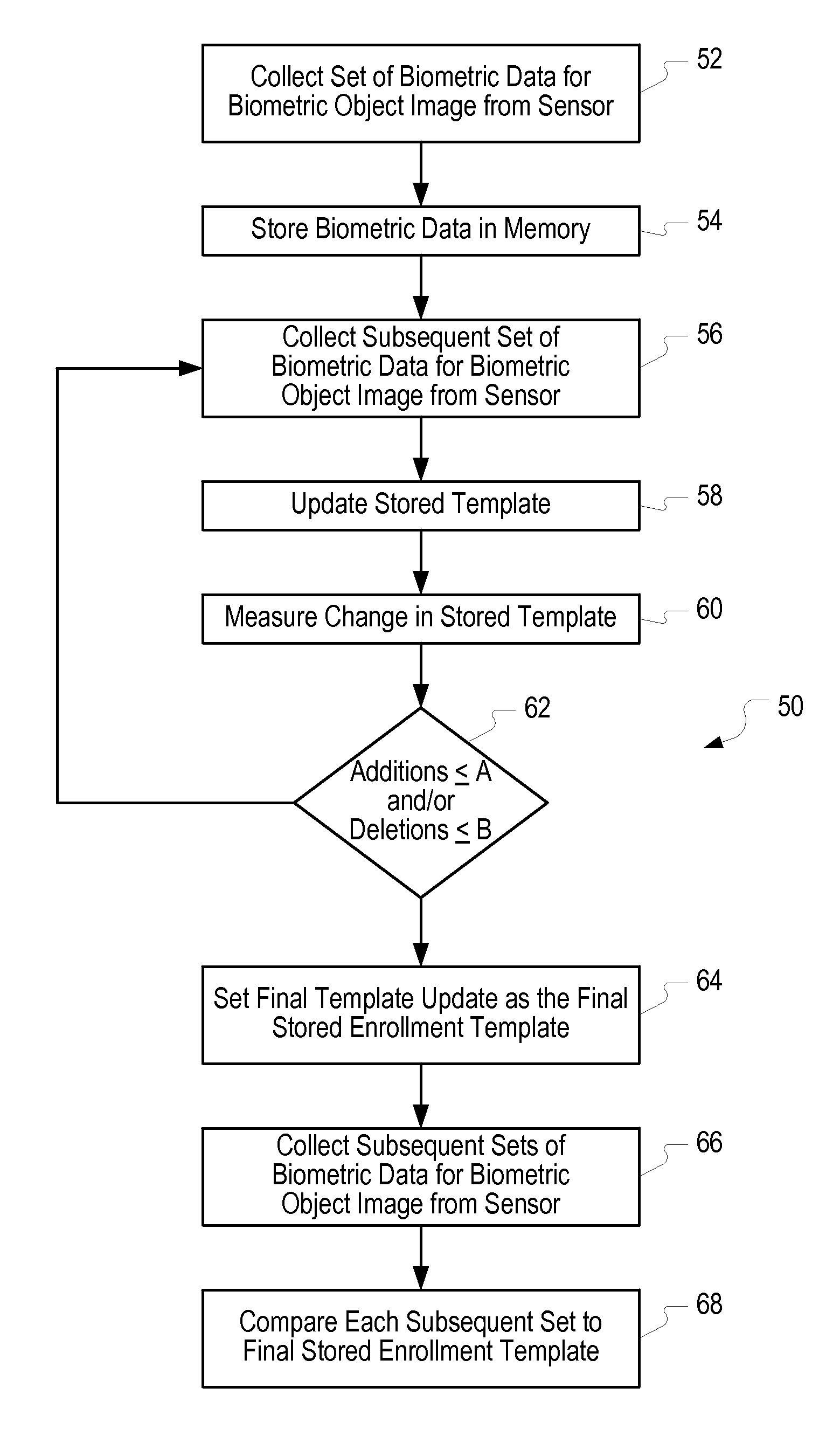
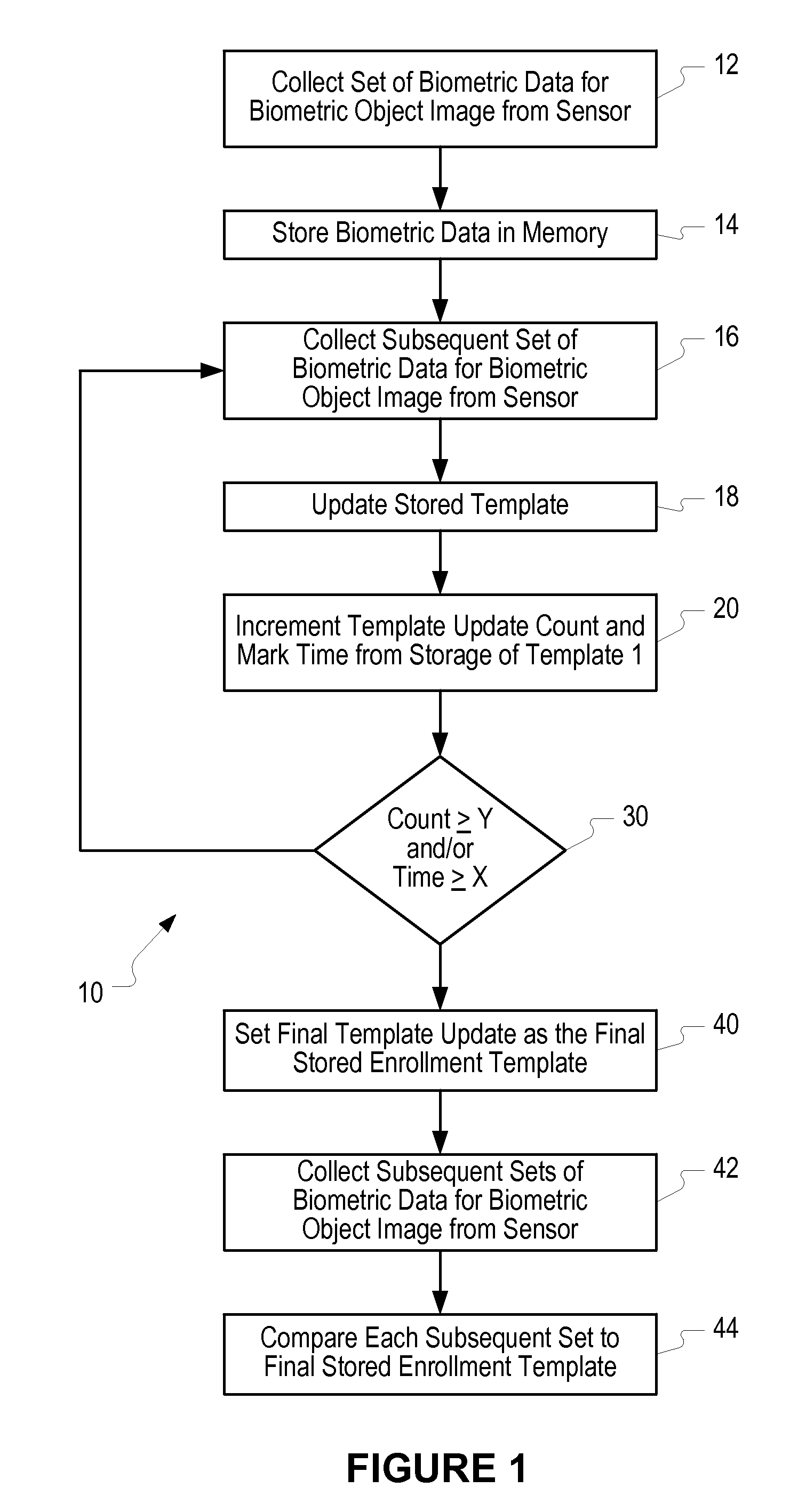
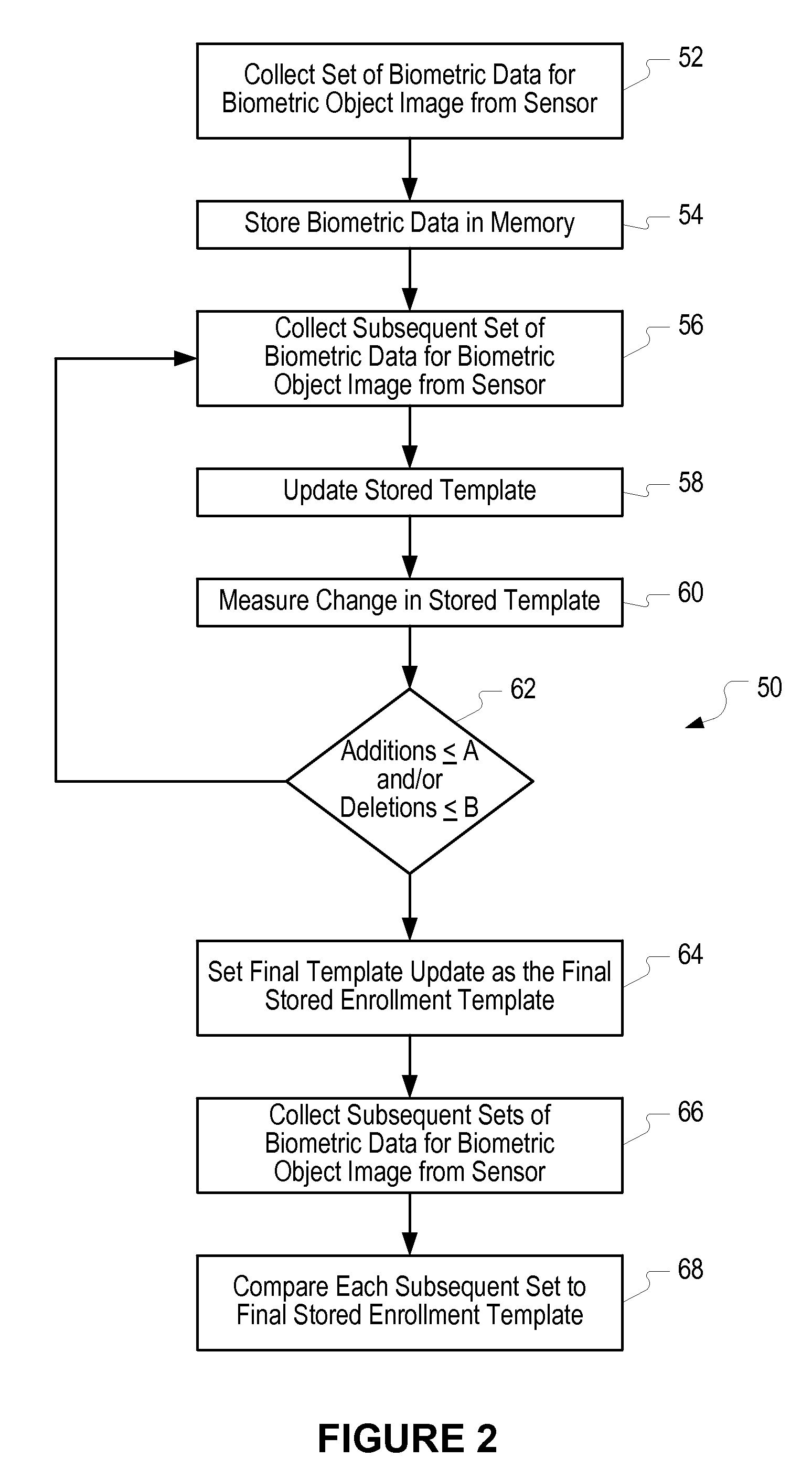
Method of and system for enrolling and matching biometric data
A system and method is disclosed for comparing biometric image data to a stored enrollment template that may comprise collecting a set of biometric image data for a biometric object image from a biometric object imaging sensor; storing the biometric object image data in a memory as an enrollment template for further comparison to find a match with subsequently imaged biometric object image data; collecting a subsequent set of biometric image data for a biometric object image from the biometric object imaging sensor; updating the enrollment template; determining if a limited enrollment window remains open; and repeating the collecting of a subsequent set of biometric data step if the enrollment window remains open. Determining if the enrollment window remains open may be by determining the existence of one of a stability indicator and an instability indicator.
Owner:SYNAPTICS INC
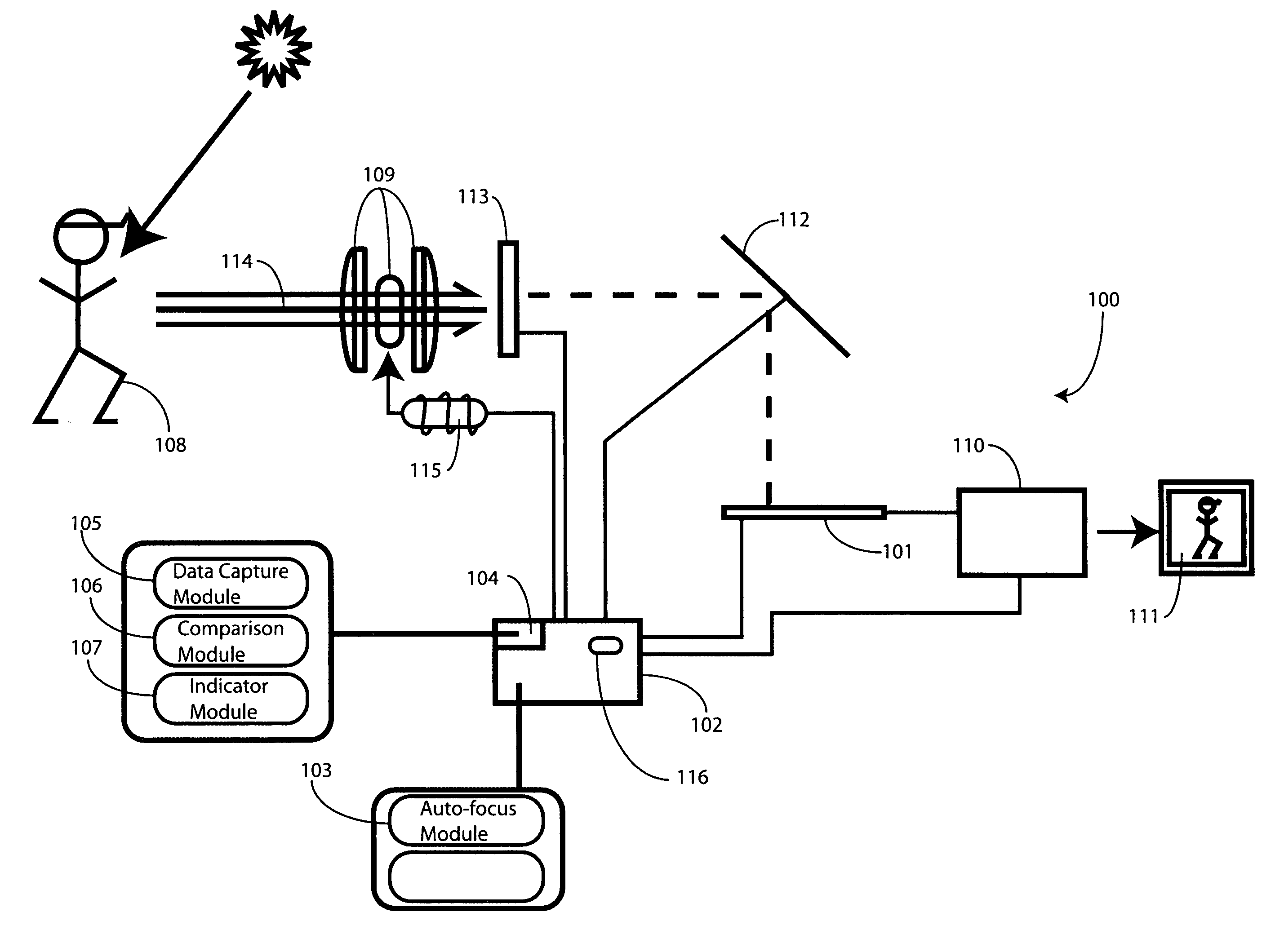
Method and Apparatus for Motion Detection in Auto-Focus Applications
InactiveUS20090273704A1Television system detailsProjector focusing arrangementFrequency spectrumComputer graphics (images)
A method (300) and image capture device (100) are provided for determining whether an image incident upon an image sensor (101) is sufficiently stable prior to executing an auto-focus process. An image stability mechanism (104) compares attribute data—such as luminance or frequency spectrum—from successive images to determine whether a change between the attribute data from image to image is within a predetermined range (710). The image stability mechanism (104) can also be configured to determine whether a scene incident upon the image sensor (101) has changed. Where the image is sufficiently stable, an indicator module (107) provides an image stability indication to a control circuit (102). The control circuit (102) can then be configured to execute the auto-focus operation when the image is sufficiently stable, or when both the scene has changed and the image is sufficiently stable.
Owner:GOOGLE TECH HLDG LLC
Method of and system for enrolling and matching biometric data
A system and method is disclosed for comparing biometric image data to a stored enrollment template that may comprise collecting a set of biometric image data for a biometric object image from a biometric object imaging sensor; storing the biometric object image data in a memory as an enrollment template for further comparison to find a match with subsequently imaged biometric object image data; collecting a subsequent set of biometric image data for a biometric object image from the biometric object imaging sensor; updating the enrollment template; determining if a limited enrollment window remains open; and repeating the collecting of a subsequent set of biometric data step if the enrollment window remains open. Determining if the enrollment window remains open may be by determining the existence of one of a stability indicator and an instability indicator.
Owner:SYNAPTICS INC
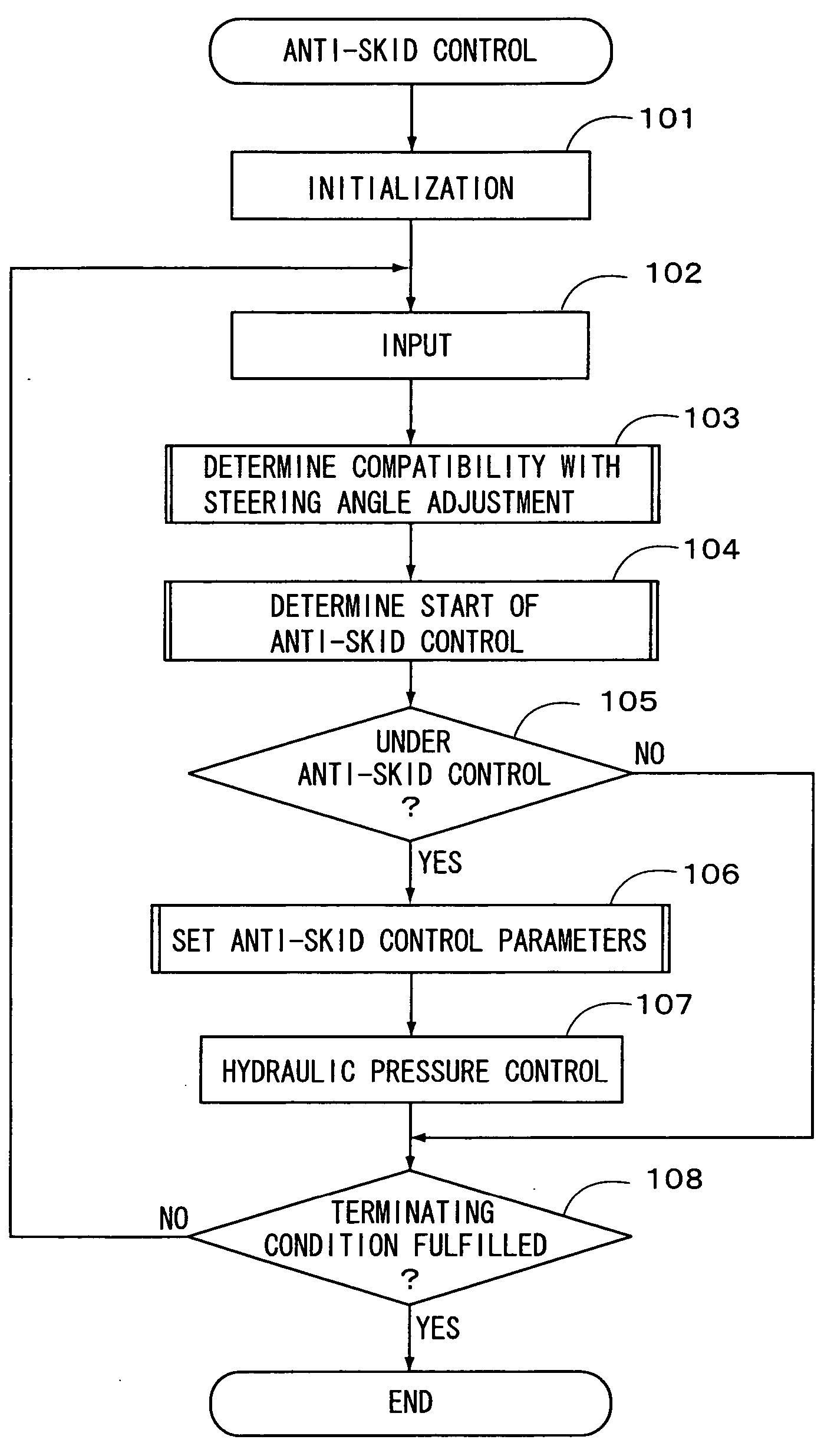
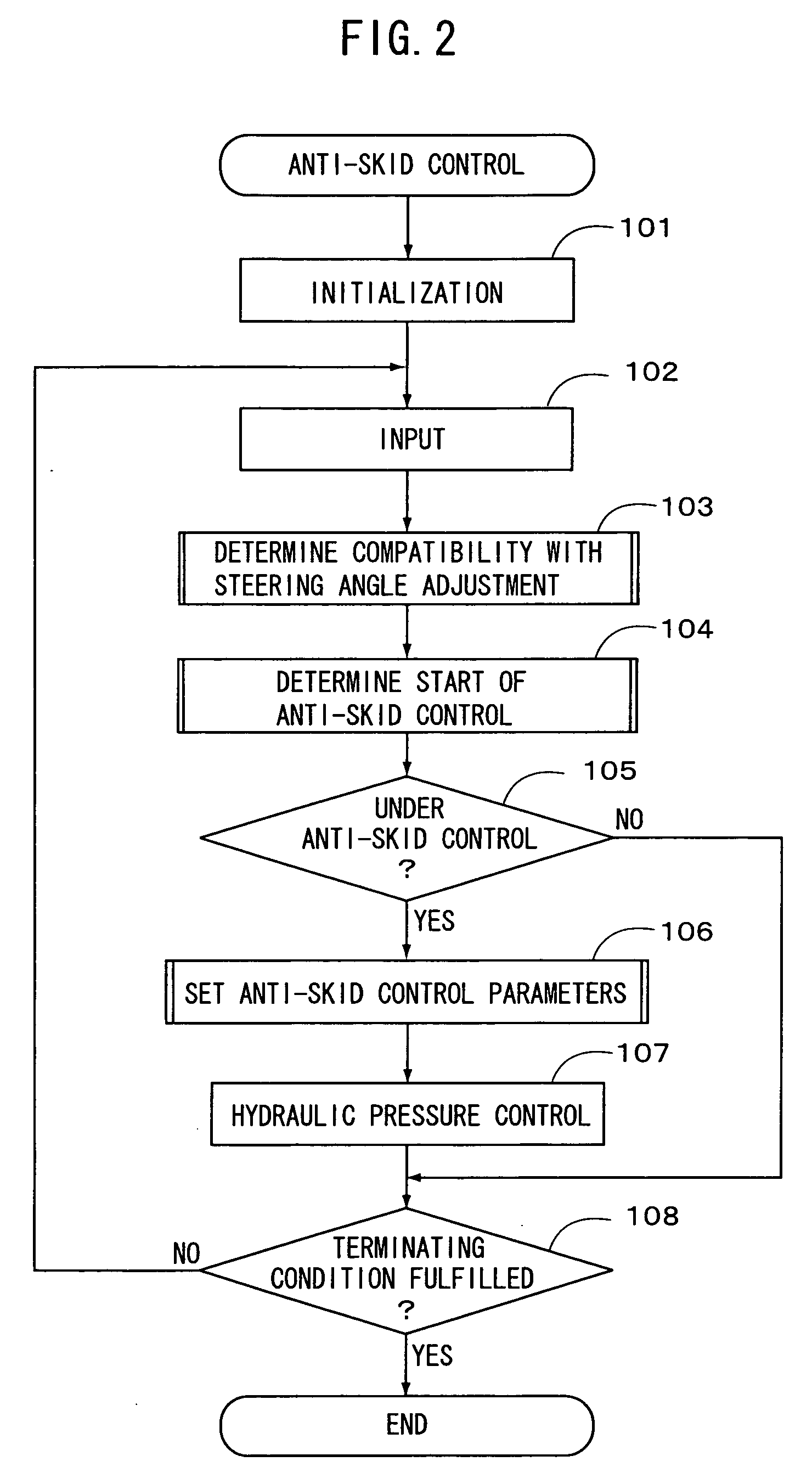
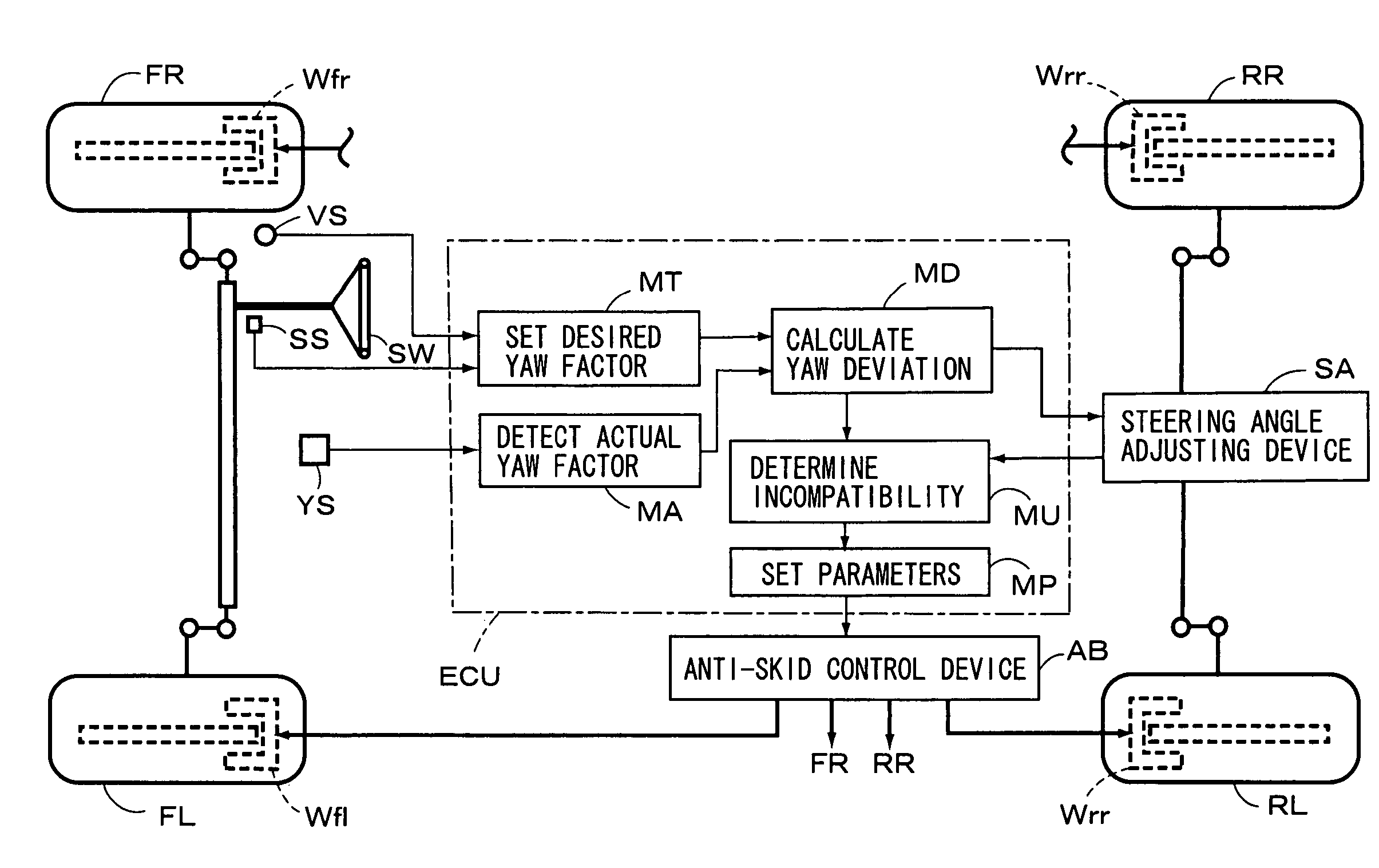
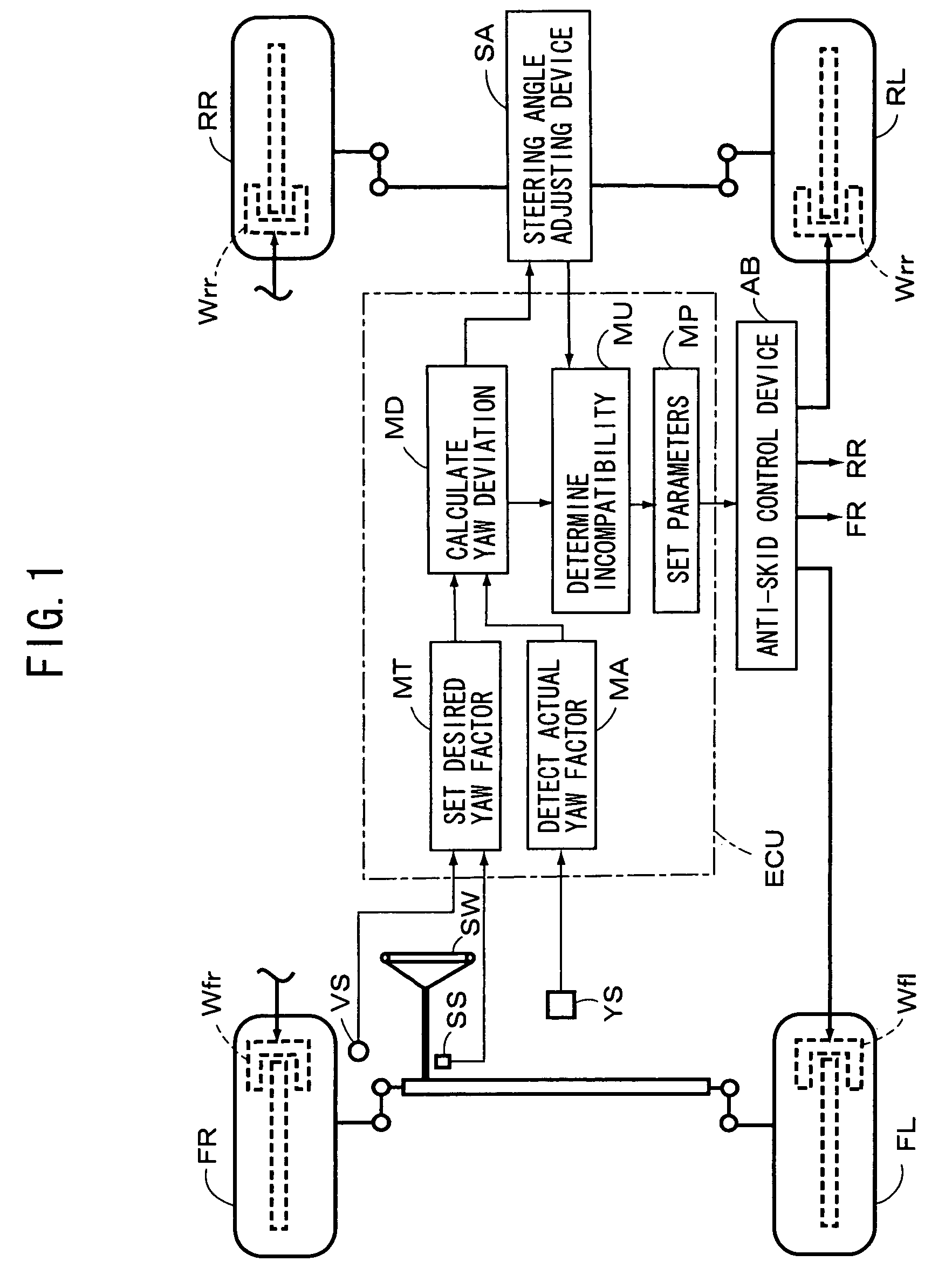
Vehicle motion control apparatus
InactiveUS20050065697A1Minimal braking distanceStabilizing vehicleBrake system interactionsSteering initiationsSteering angleStability indicating
A vehicle motion control apparatus is provided with an anti-skid control device and a steering angle adjusting device, which adjusts the steering angle of at least one of the front and rear wheels to cancel a yaw deviation between a desired yaw factor and an actual yaw factor, to be substantially zero. An incompatibility between the devices is determined on the basis of a state of the wheel adjusted by the steering angle adjusting device to cancel the yaw deviation. And, a predetermined parameter provided between a vehicle stability directive parameter and a brake directive parameter is set on the basis of the incompatibility. Then, the anti-skid control device controls the braking force applied to each wheel of the vehicle on the basis of the predetermined parameter. Consequently, the anti-skid control giving importance to the braking force can be performed, as long as the steering angle adjusting device is operative.
Owner:JTEKT CORP +1
Image processing apparatus, display control method and program
ActiveCN102867169AAvoid confusionImage analysis2D-image generationPattern recognitionImaging processing
Aspects of the present invention include an apparatus comprising a recognition unit configured to recognize real object in an image. The apparatus may further comprise a determining unit configured to determine a stability indicator indicating a stability of the recognition, and a display control unit configured to modify a display of a virtual object according to the stability indicator.
Owner:SONY CORP
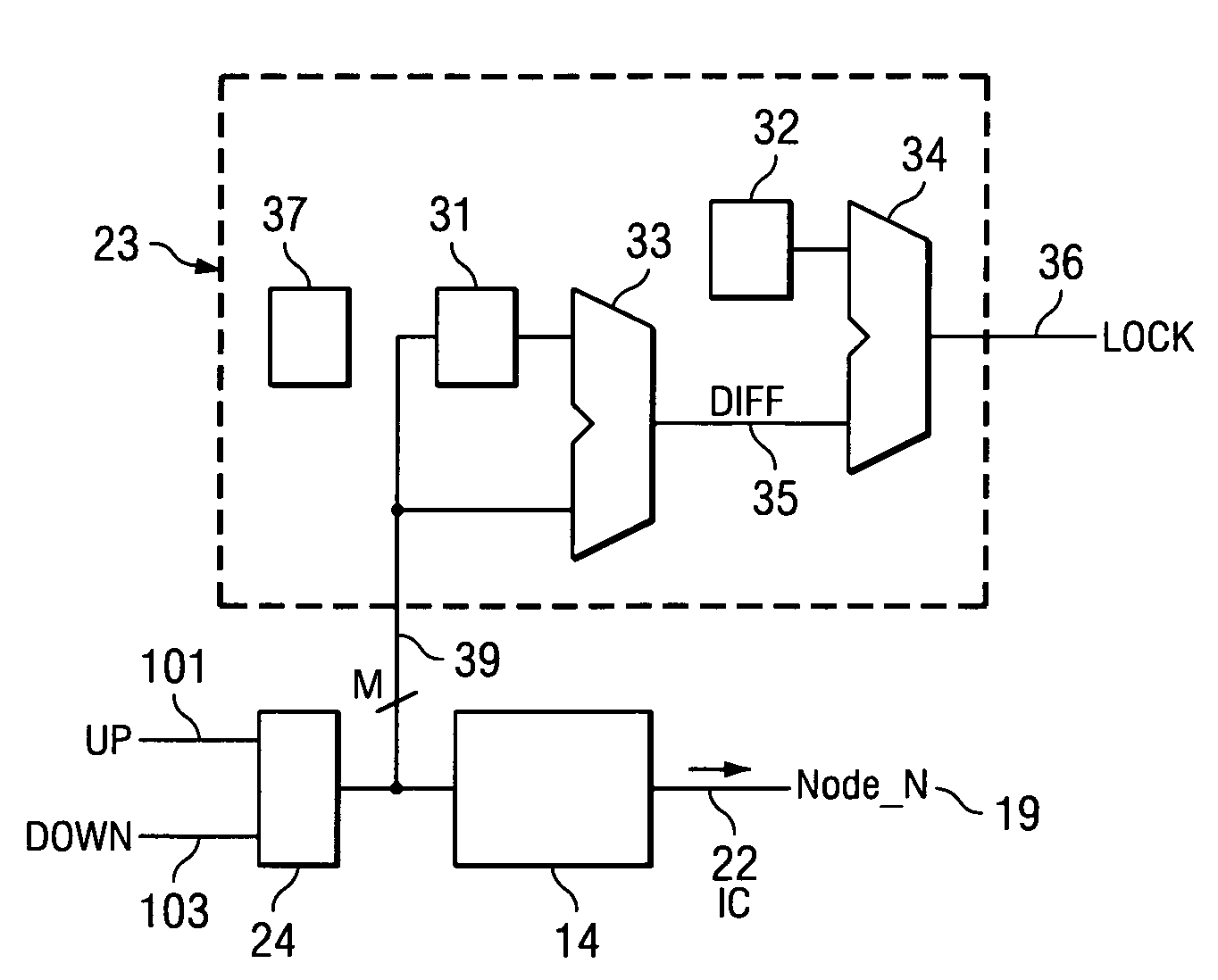
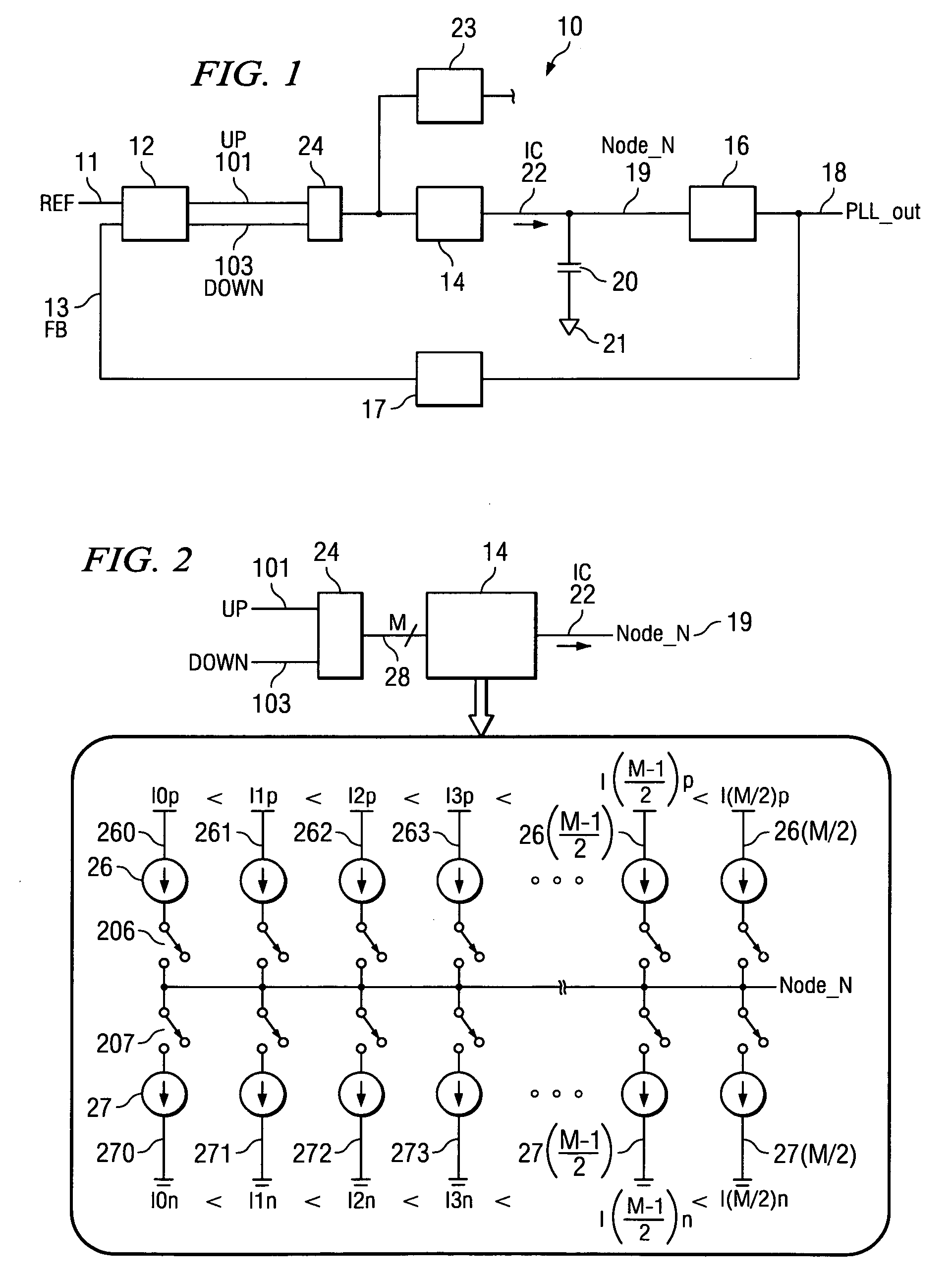
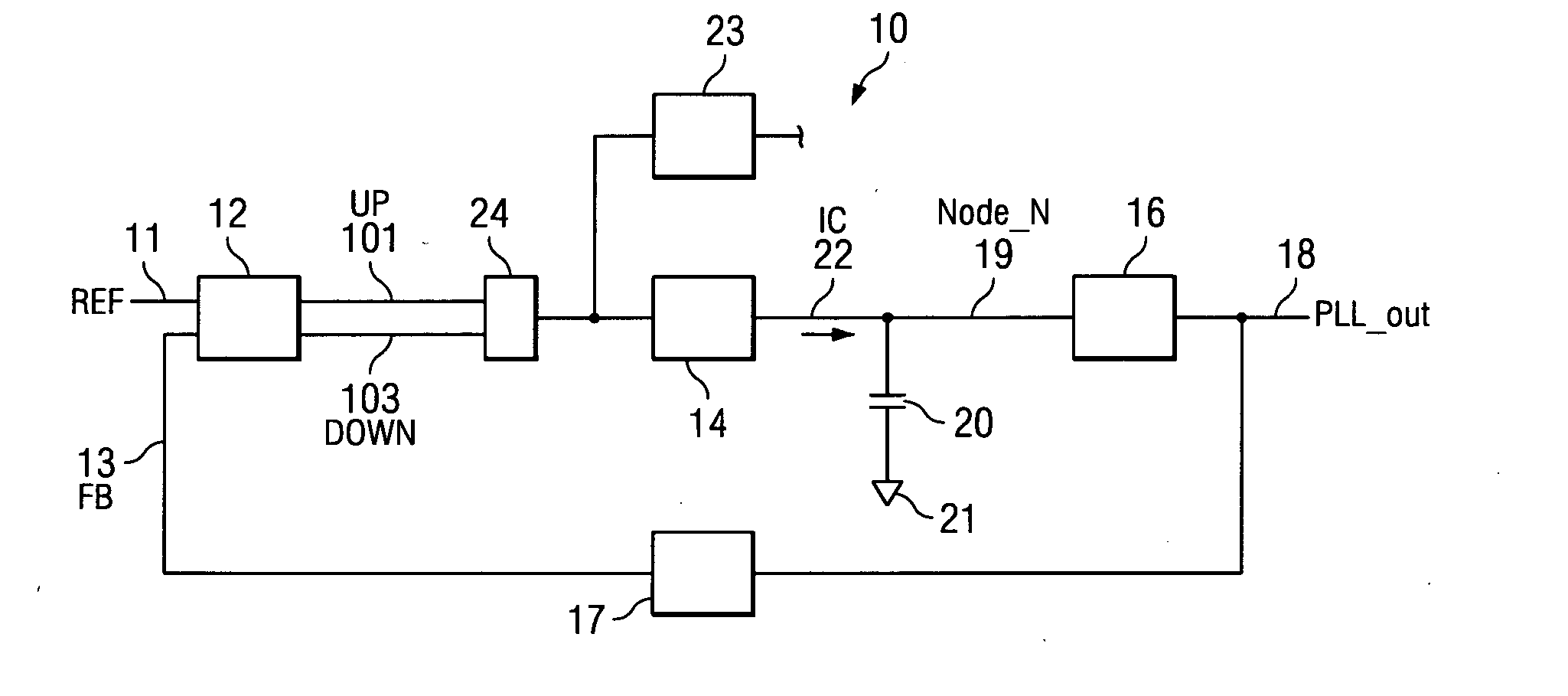
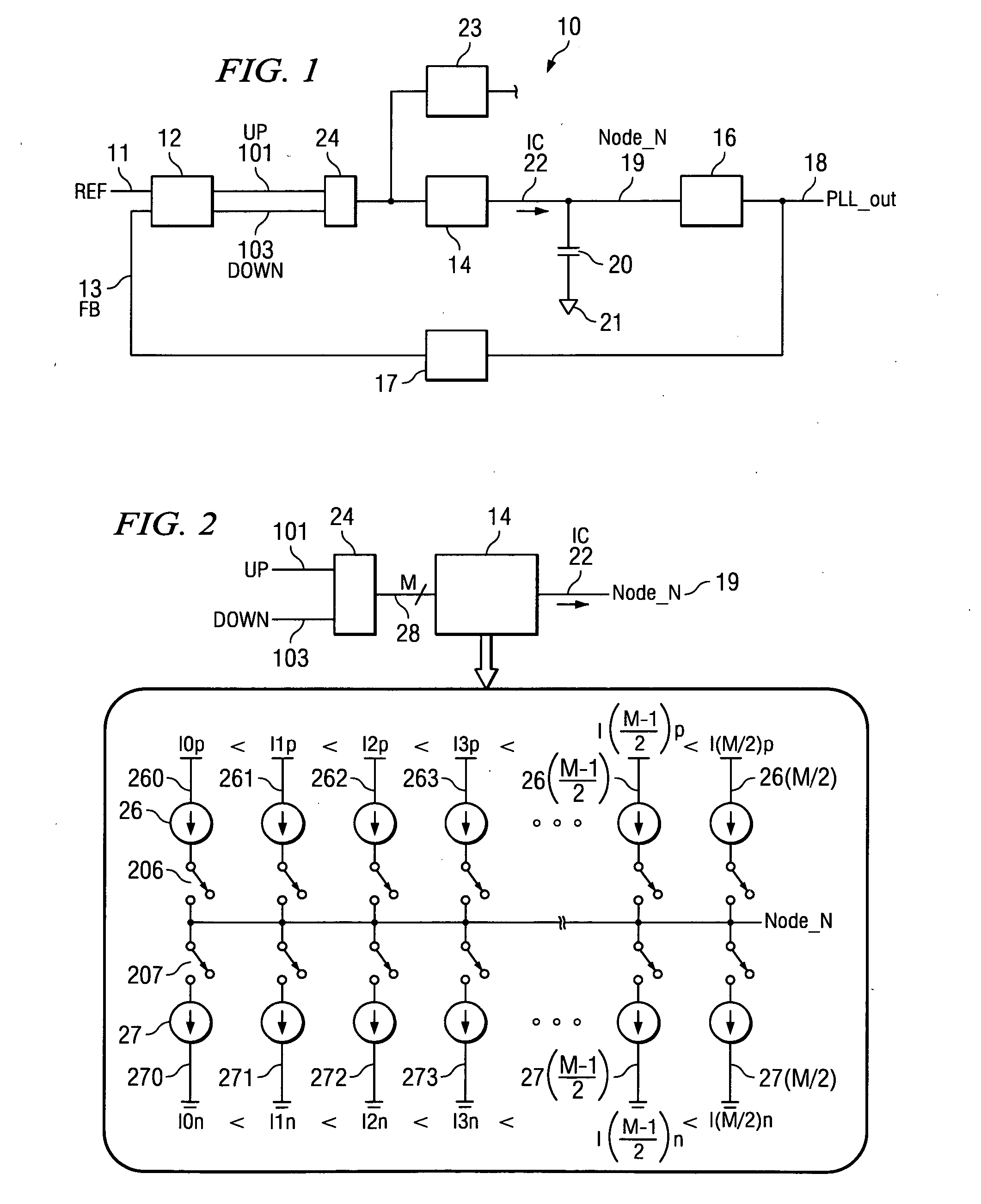
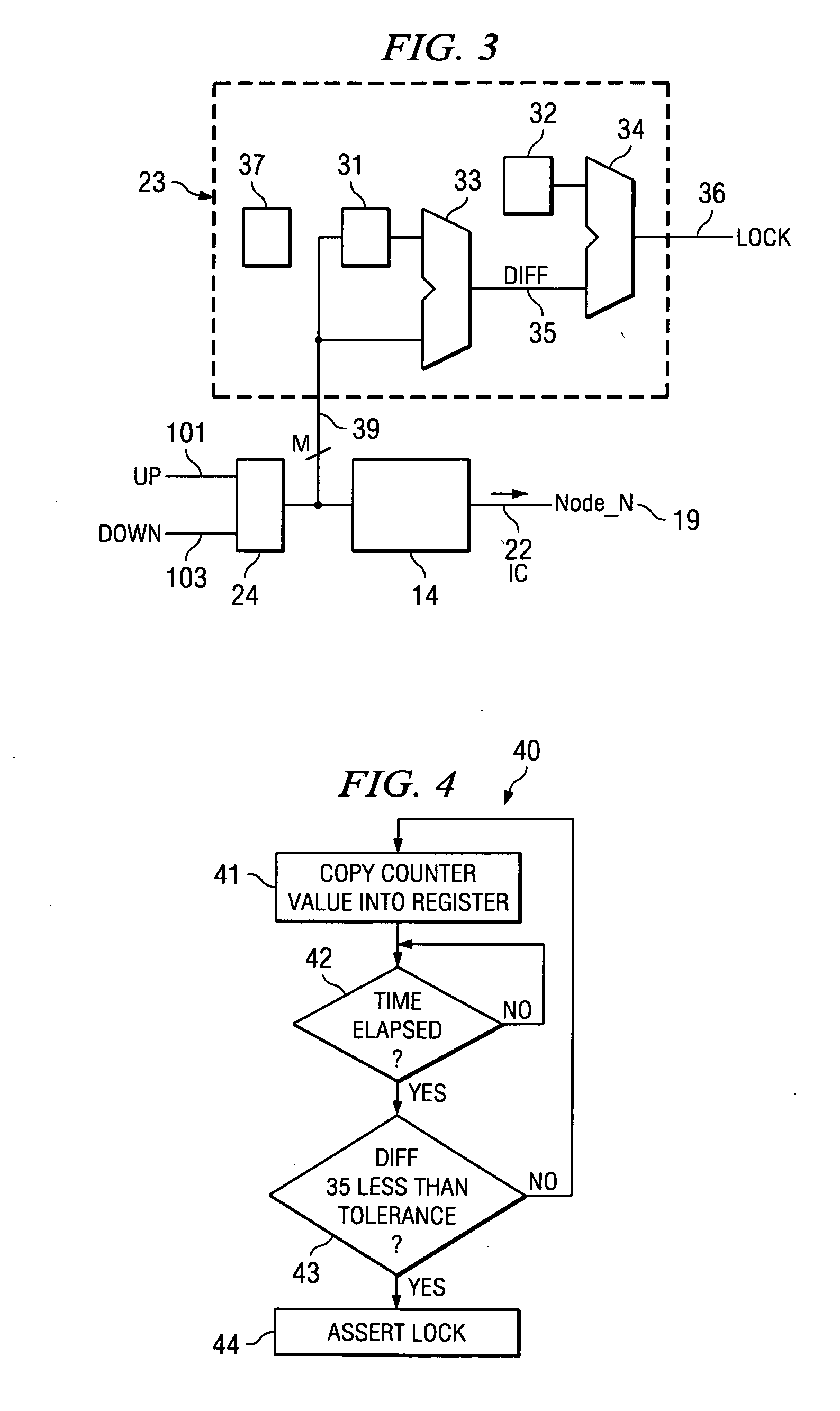
System and method for lock detection of a phase-locked loop circuit
InactiveUS7268629B2Reduction in number of erroneous indicationReduce such erroneous indicationPulse automatic controlOscillator tubesPhase locked loop circuitProcessor register
Systems and methods for detecting phase-locked loop circuit lock. In particular, a lock detector configured to detect PLL stability for a user-defined period of time prior to asserting a PLL-lock-detected output. Stability may be indicated by a counter inserted into a PLL circuit and arranged between a phase-frequency detector and a charge pump. Because the counter value is acted upon by the phase-frequency detector, PLL lock is indicated by counter value stability. The digital counter value may be provided to a digital charge pump and a lock detector simultaneously. The lock detector includes registers and difference detectors to determine when the difference between counter values is below a user-defined tolerance. The lock detector may include a variable timer to avoid false indications of lock which may occur when counter values are sampled with the same frequency as a fluctuation frequency of the counter value.
Owner:KK TOSHIBA
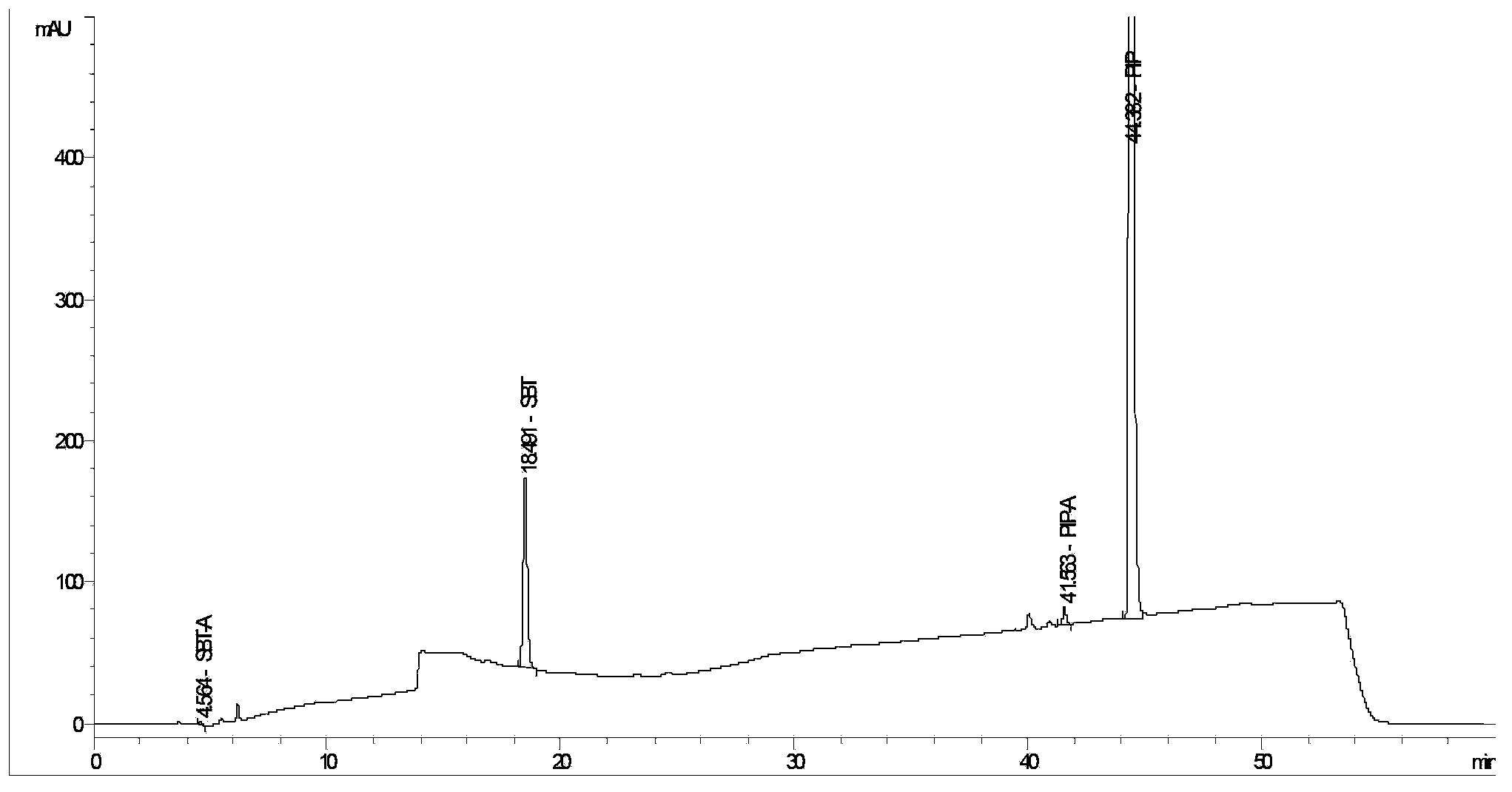
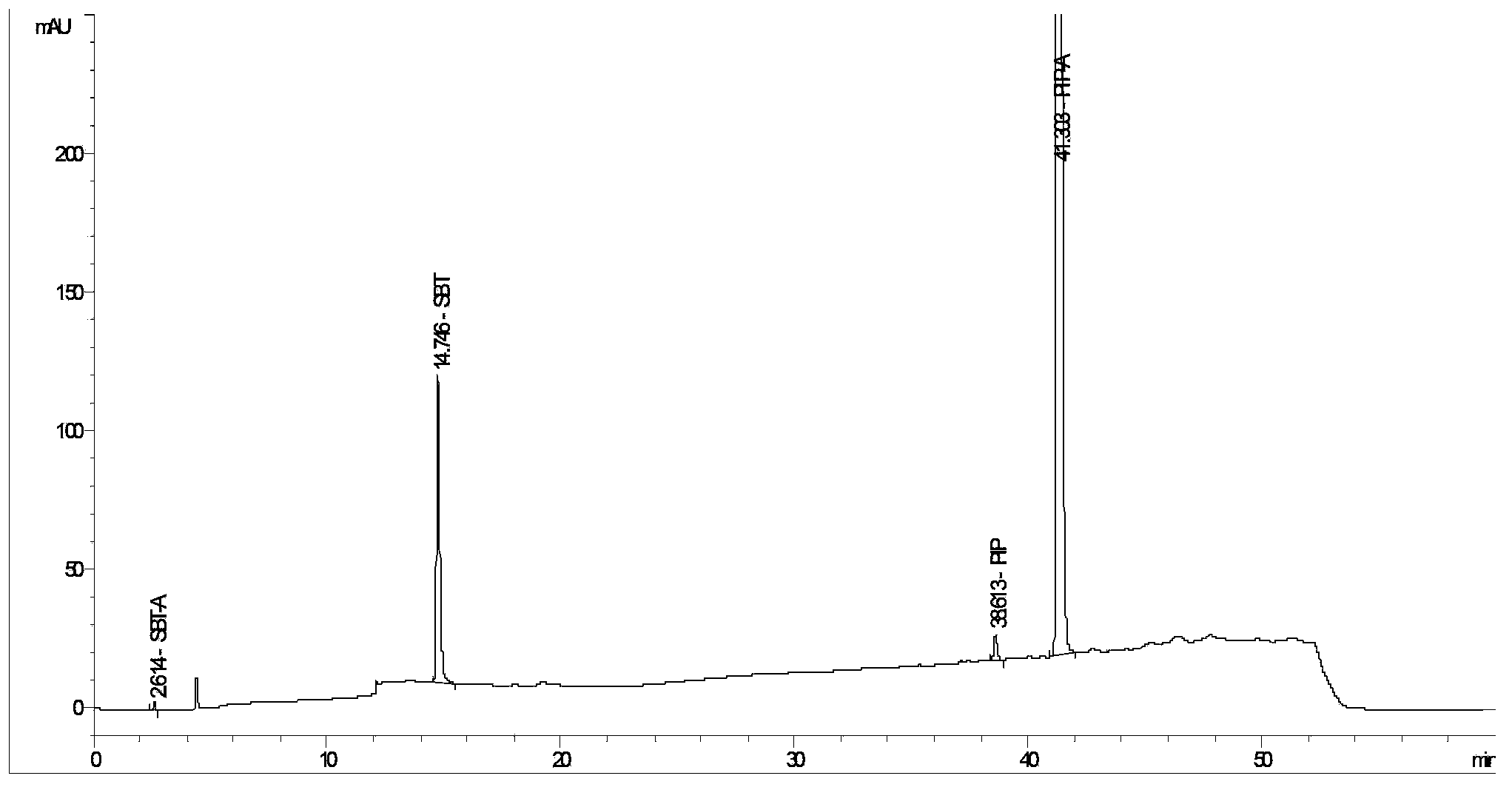
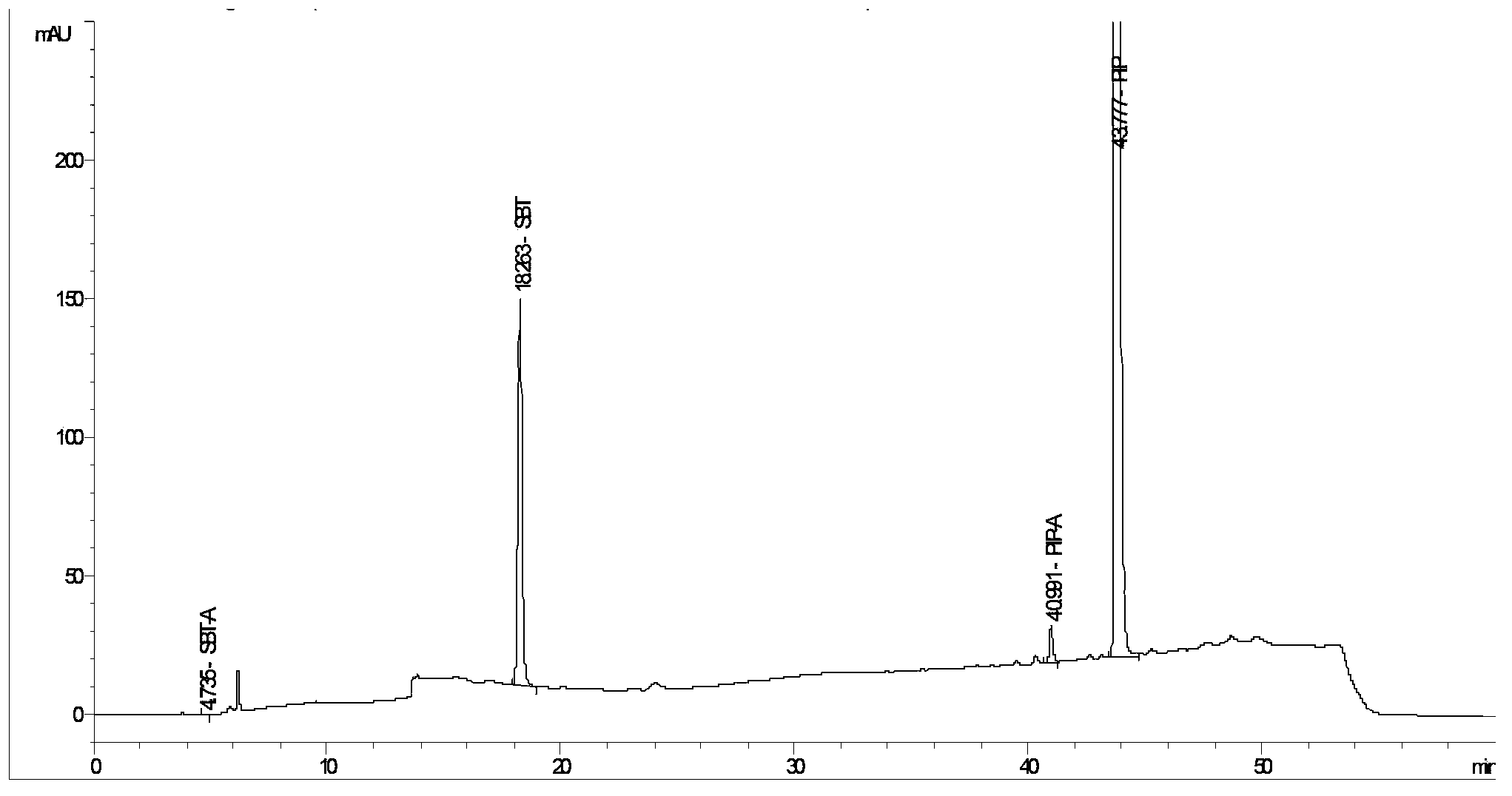
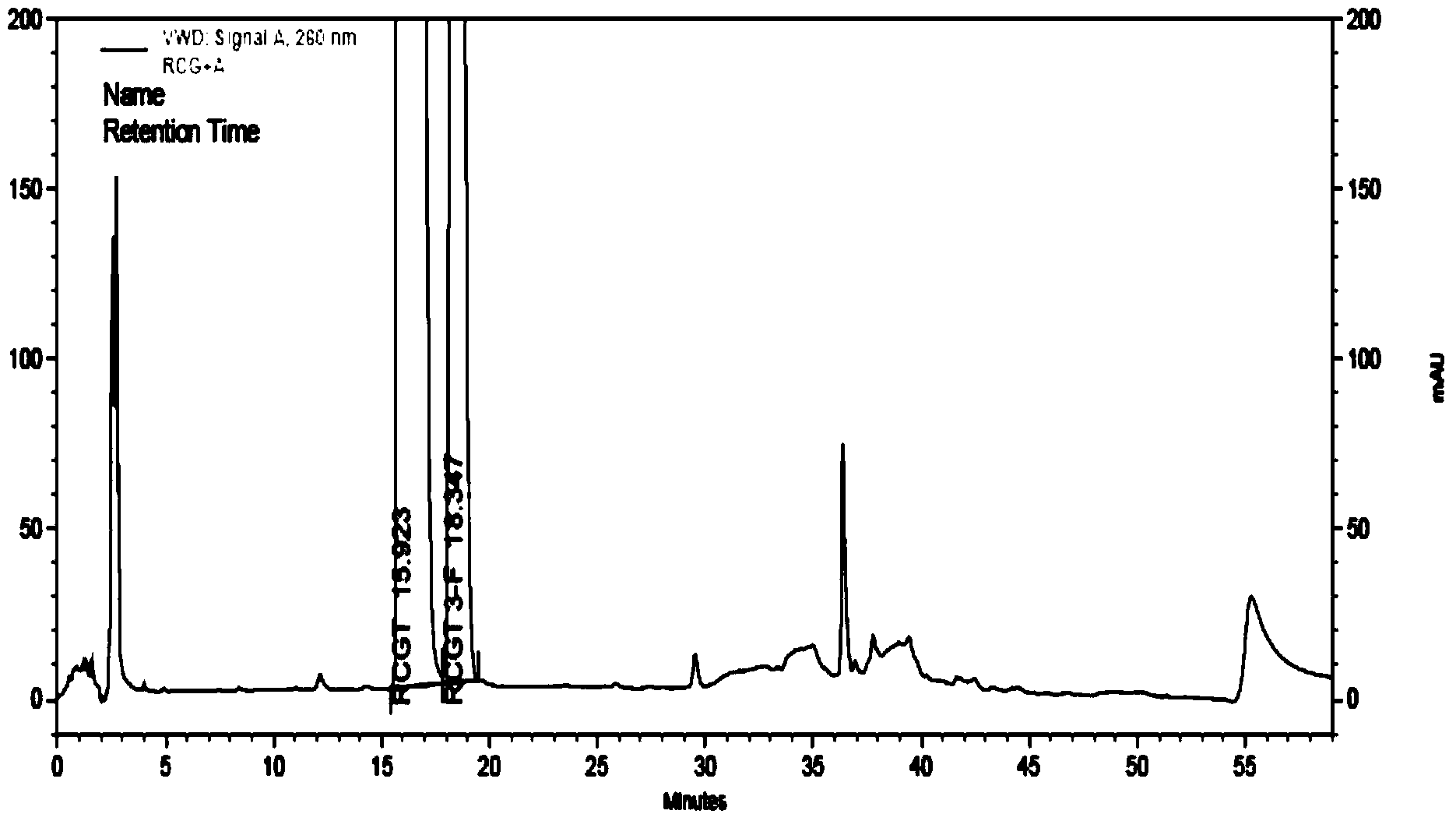
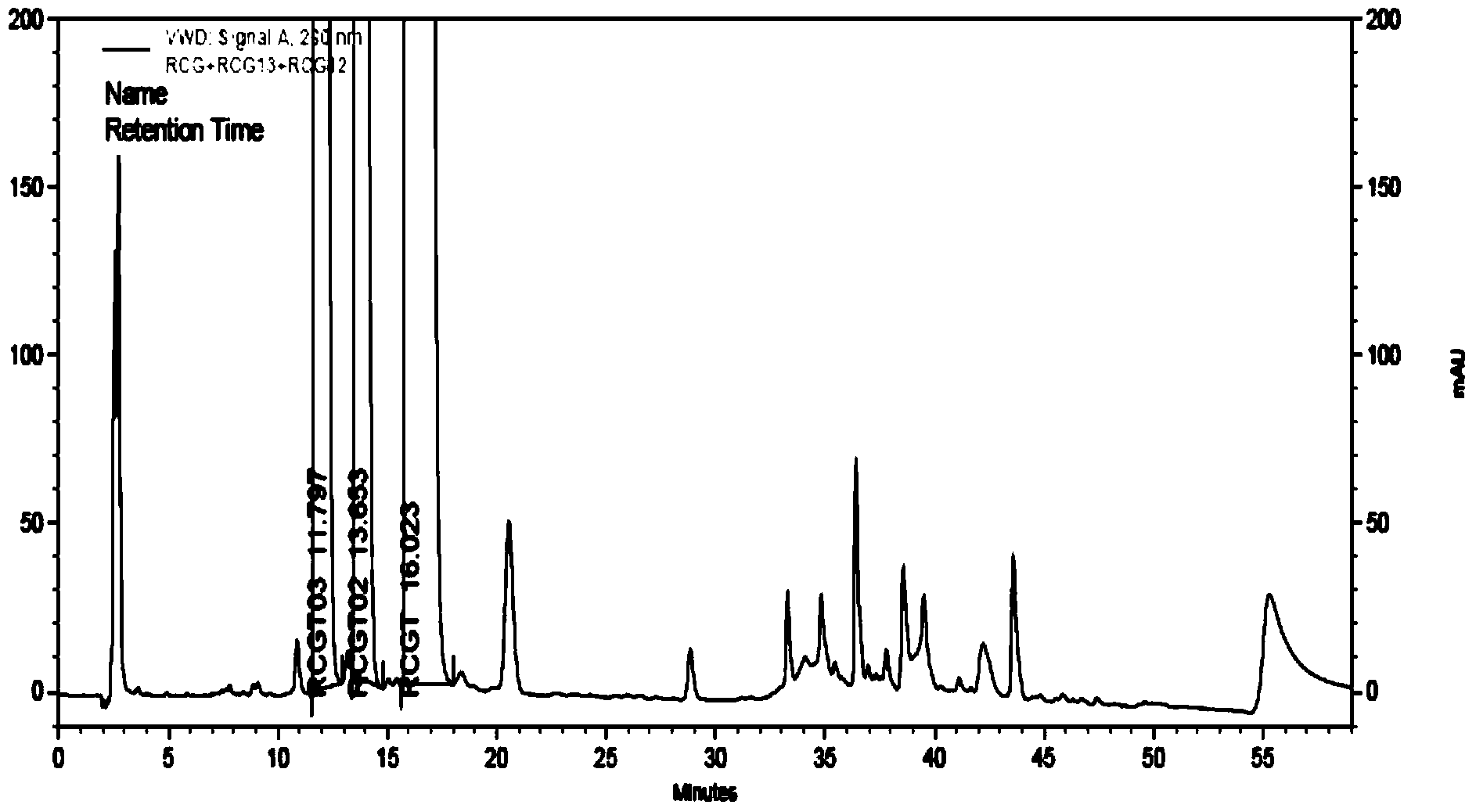
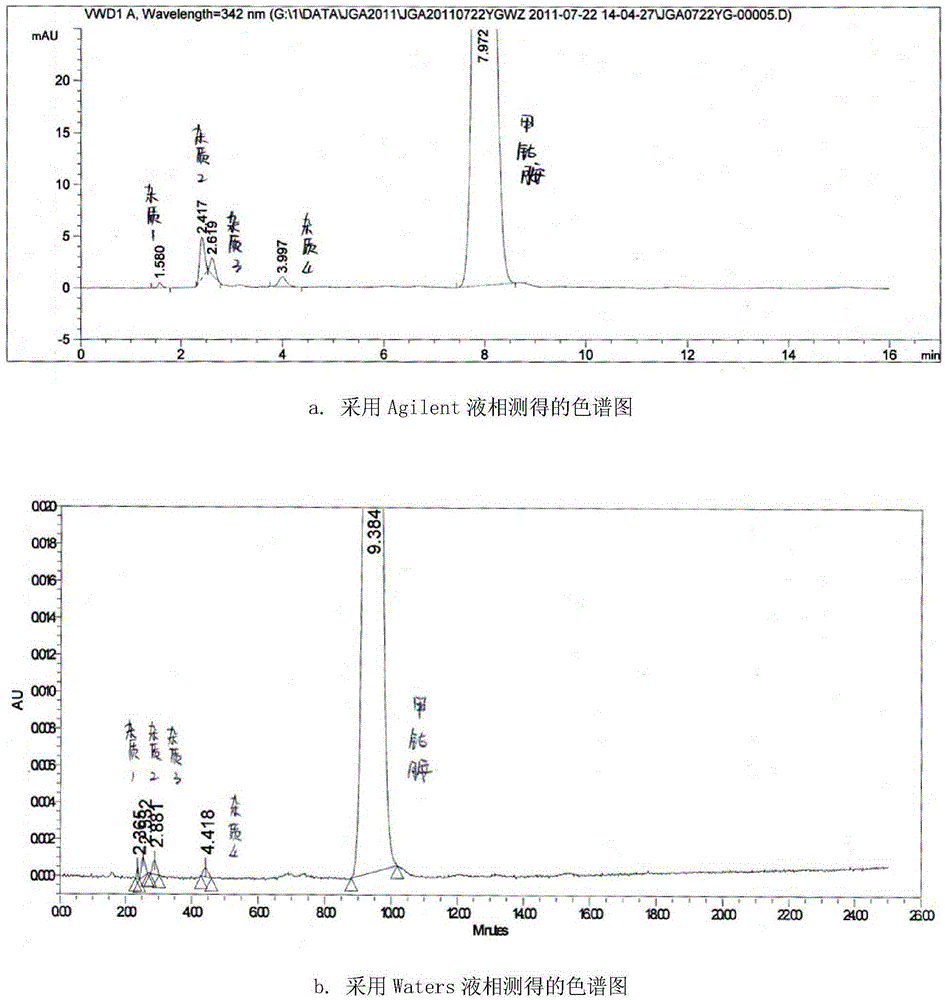
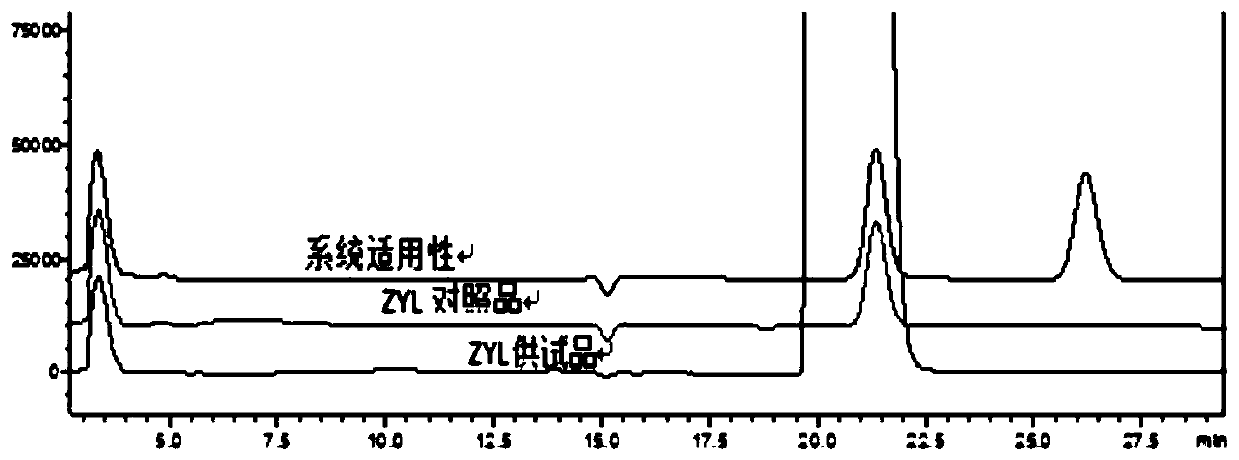
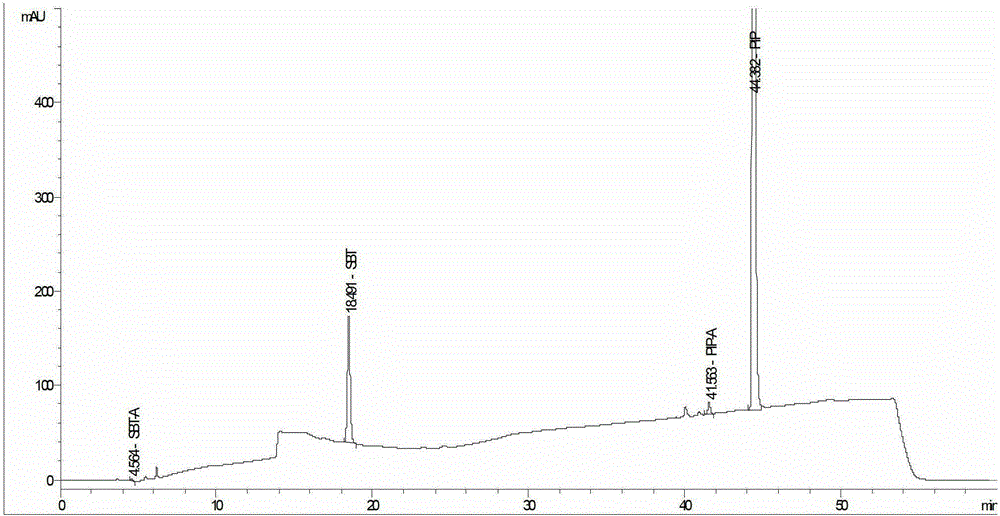
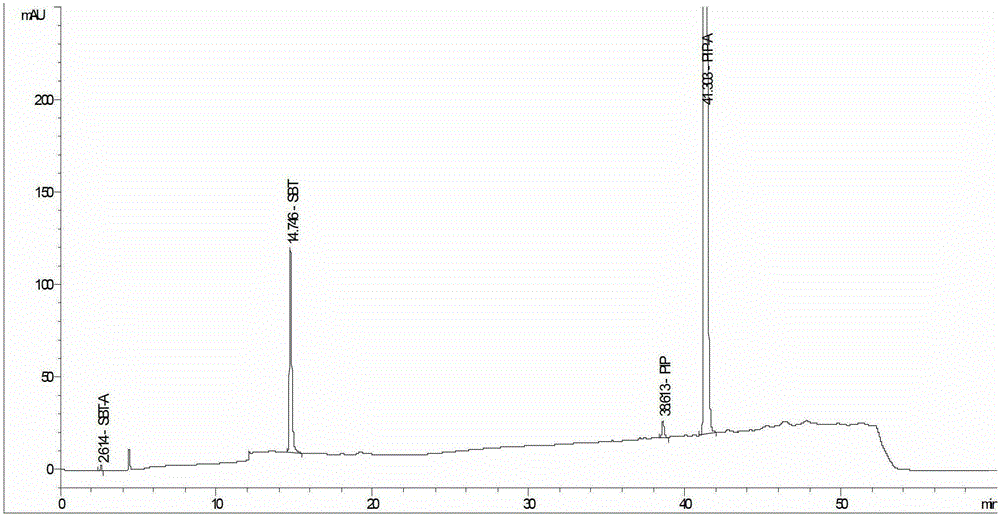
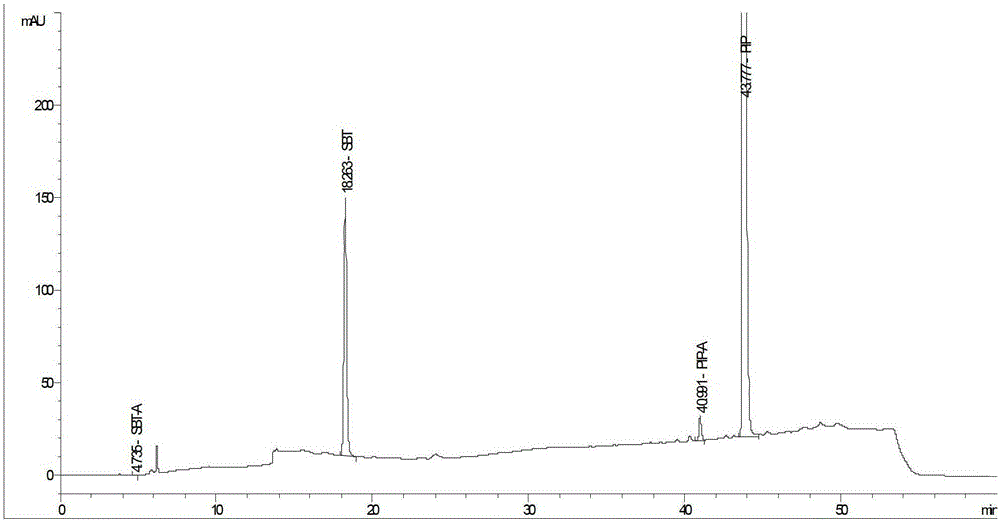
Method for detecting related substances in piperacillin sodium and sulbactum sodium for injection
ActiveCN104215697ARealize determinationEfficient separationComponent separationSilanesStability indicating
The invention provides a method for detecting related substances in piperacillin sodium and sulbactum sodium for injection. The method adopts an octadecyl silane bonded silica-gel chromatographic column to carry out gradient elution so as to rapidly and precisely complete the detection on related substances in piperacillin sodium and sulbactum sodium. In the obtained chromatogram, the main related substance (2S)-2-amino-3-methyl-3-sulfinobutyric acid and piperacillin penicilloic acid are well separated, piperacillin and sulbactum are also well separated from other related substances, and the separation degree is more than 1.5. The degradation researches and methodology on piperacillin sodium and sulbactum sodium show that the provided method has a stability indicating function, and thus the detection method can be used to control the limits of impurities in piperacillin sodium and sulbactum sodium, and can also be used to control the quality of piperacillin sodium and sulbactum sodium for injection. The method has the advantages of convenient operation and low cost, and has a good economic profit and promotion prospect.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
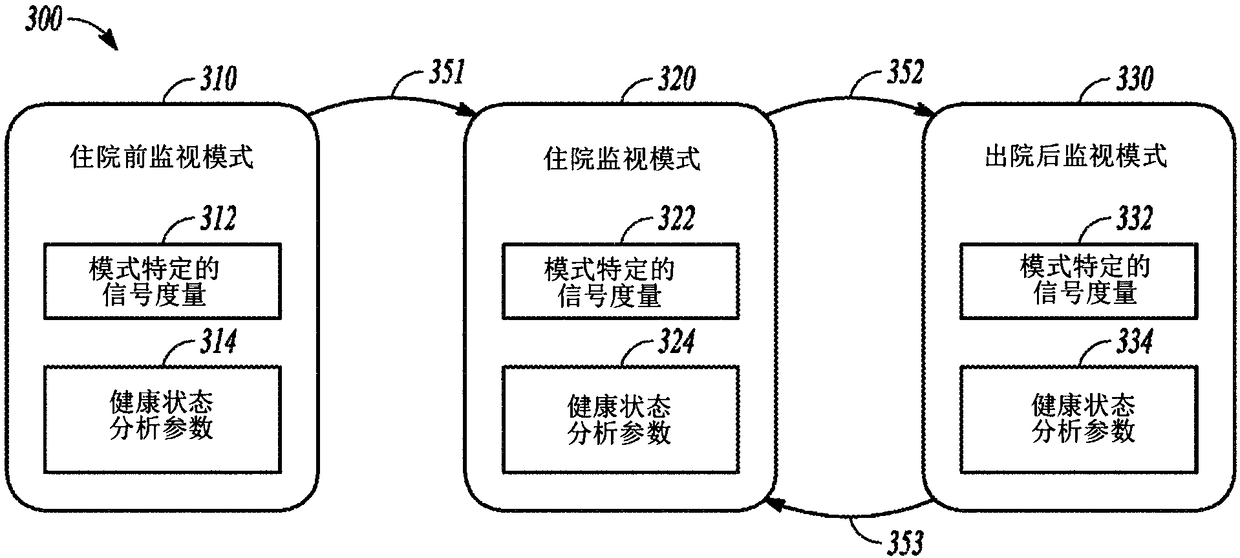
Systems and methods for patient monitoring
The present invention discloses systems and methods for monitoring patients with a chronic disease such as heart failure. The system may include a physiological sensor circuit to sense physiological signals and generate signal metrics from the physiological signals. The system may include a health status analyzer circuit to use the signal metrics to generate one or more stability indicators of patient health status, such as stability of heart failure status. The system may additionally generate one or more health status indicators indicating patient health status such as heart failure progression. A patient disposition decision may be generated using the health status indicators and the stability indicators to provide an indication of readiness for patient discharge from or a risk of admission to a hospital.
Owner:CARDIAC PACEMAKERS INC
Vehicle motion control apparatus
InactiveUS7212903B2Minimal braking distanceVehicle stabilityBrake system interactionsAnalogue computers for trafficSteering angleStability indicating
A vehicle motion control apparatus is provided with an anti-skid control device and a steering angle adjusting device, which adjusts the steering angle of at least one of the front and rear wheels to cancel a yaw deviation between a desired yaw factor and an actual yaw factor, to be substantially zero. An incompatibility between the devices is determined on the basis of a state of the wheel adjusted by the steering angle adjusting device to cancel the yaw deviation. And, a predetermined parameter provided between a vehicle stability directive parameter and a brake directive parameter is set on the basis of the incompatibility. Then, the anti-skid control device controls the braking force applied to each wheel of the vehicle on the basis of the predetermined parameter. Consequently, the anti-skid control giving importance to the braking force can be performed, as long as the steering angle adjusting device is operative.
Owner:JTEKT CORP +1
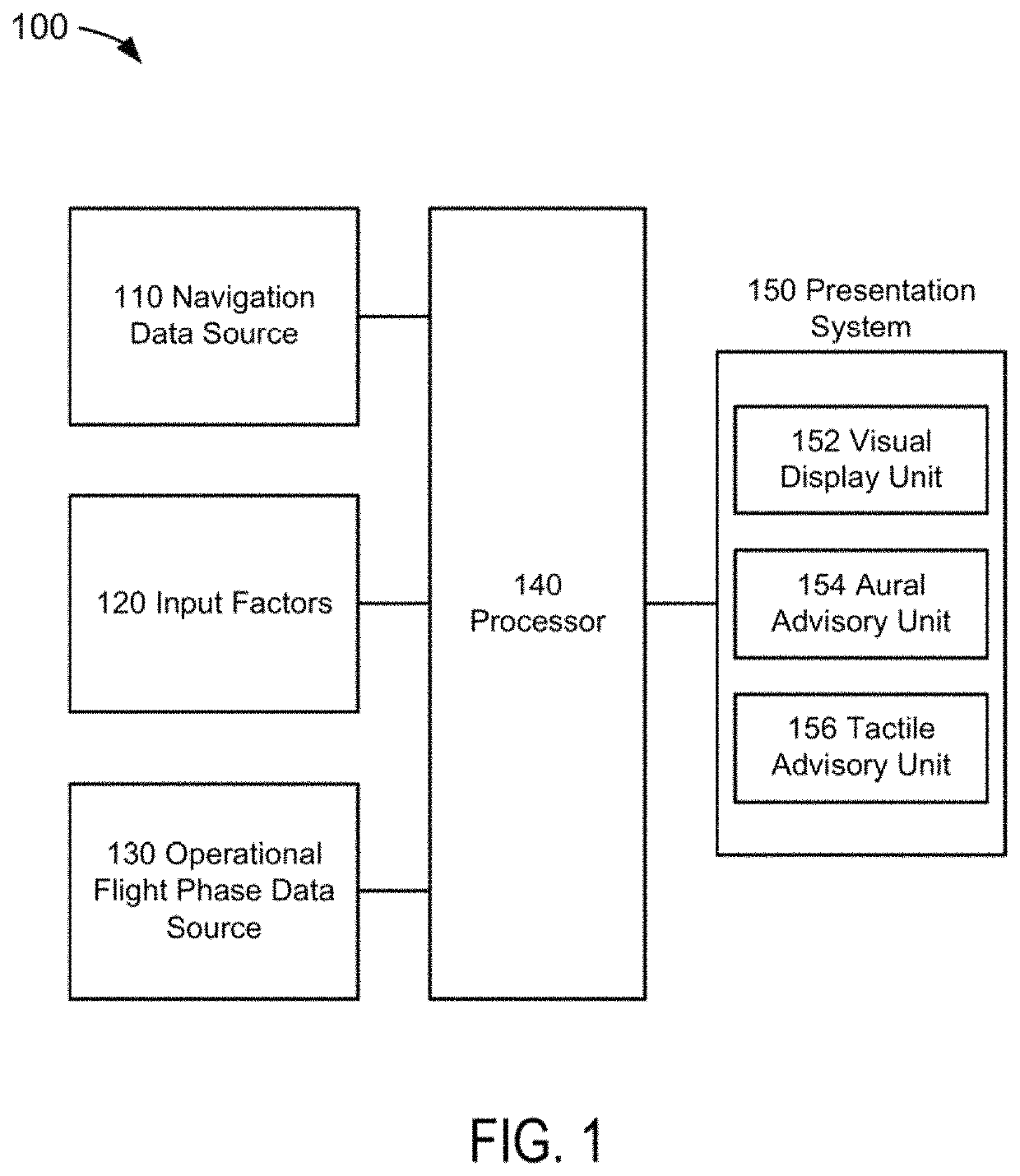
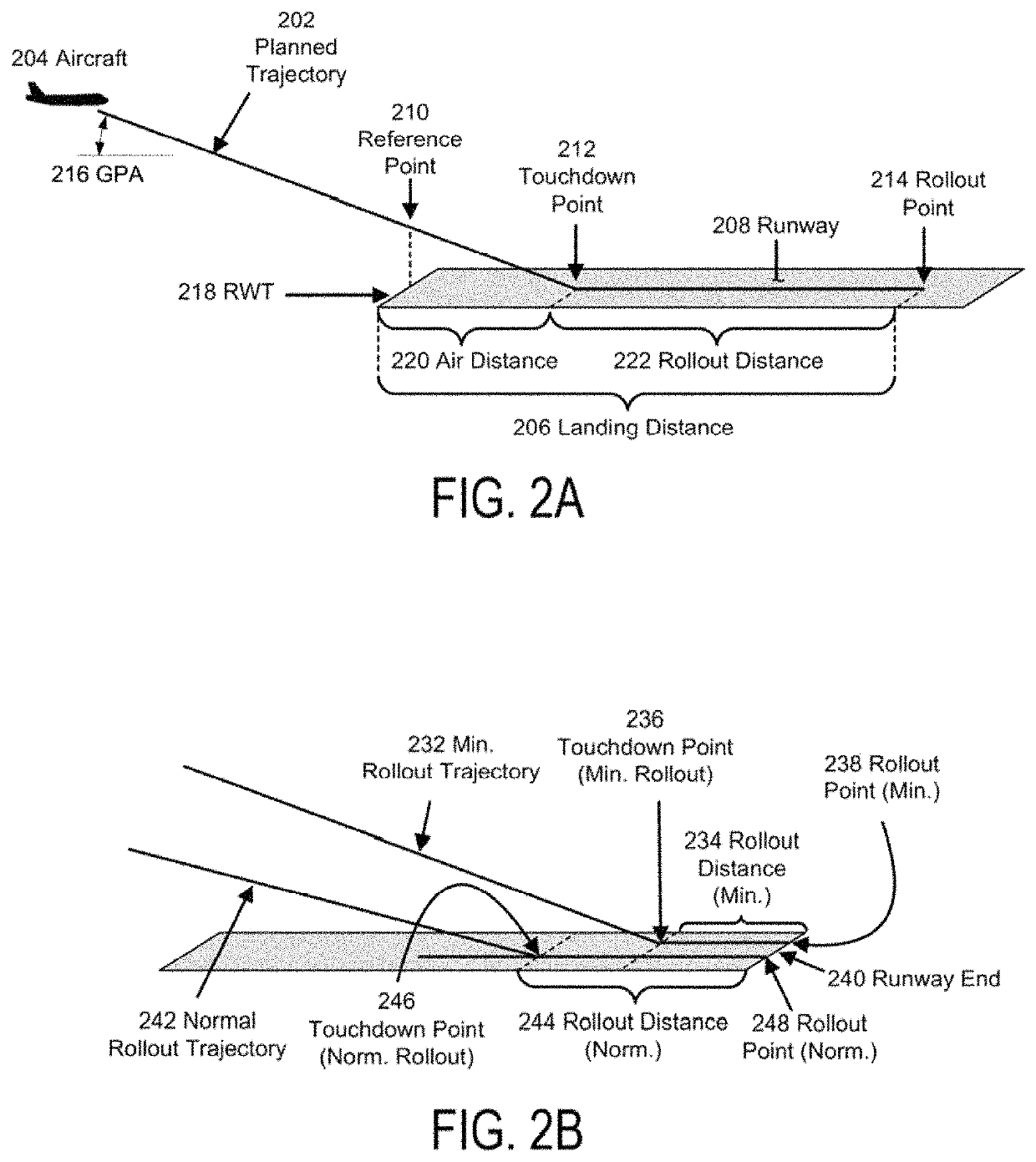
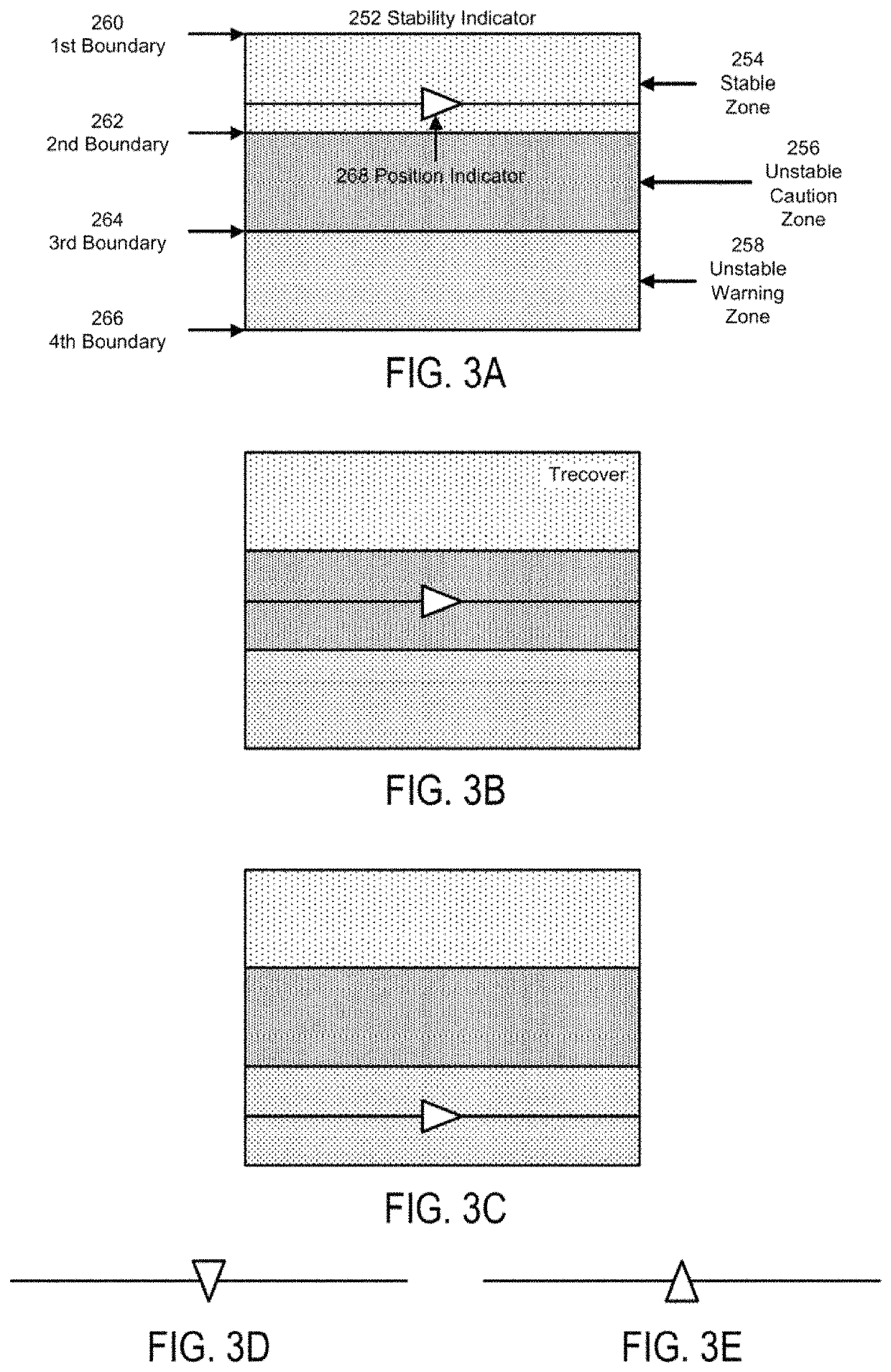
Flight phase stability indicator system and method
ActiveUS10514707B1Enhanced Situational AwarenessParachutesNavigation instrumentsDisplay deviceStability indicating
A flight phase stability indicator system and method for generating and presenting stability indicator(s) are disclosed. A processor receives flight phase data representative of an identification of a current operational flight phase; receives navigation data comprised of aircraft position, a planned flight trajectory, and runway data if runway data is needed for the current operational flight phase; determines stability data representative of a stability of the current operational flight phase based upon at least the navigation data; generates presentation data responsive to the determination and representative of the flight phase stability indicator and / or presentation(s) of flight phase stability; and presents the indicator represented in the presentation data on or through a presentation unit comprised of a visual display, aural advisory unit, and / or tactile advisory unit.
Owner:ROCKWELL COLLINS INC
System and method for lock detection of a phase-locked loop circuit
InactiveUS20060267642A1Reduction in number of erroneous indicationReduce such erroneous indicationPulse automatic controlOscillator tubesPhase locked loop circuitPhase frequency detector
Systems and methods for detecting phase-locked loop circuit lock. In particular, a lock detector configured to detect PLL stability for a user-defined period of time prior to asserting a PLL-lock-detected output. Stability may be indicated by a counter inserted into a PLL circuit and arranged between a phase-frequency detector and a charge pump. Because the counter value is acted upon by the phase-frequency detector, PLL lock is indicated by counter value stability. The digital counter value may be provided to a digital charge pump and a lock detector simultaneously. The lock detector includes registers and difference detectors to determine when the difference between counter values is below a user-defined tolerance. The lock detector may include a variable timer to avoid false indications of lock which may occur when counter values are sampled with the same frequency as a fluctuation frequency of the counter value.
Owner:KK TOSHIBA
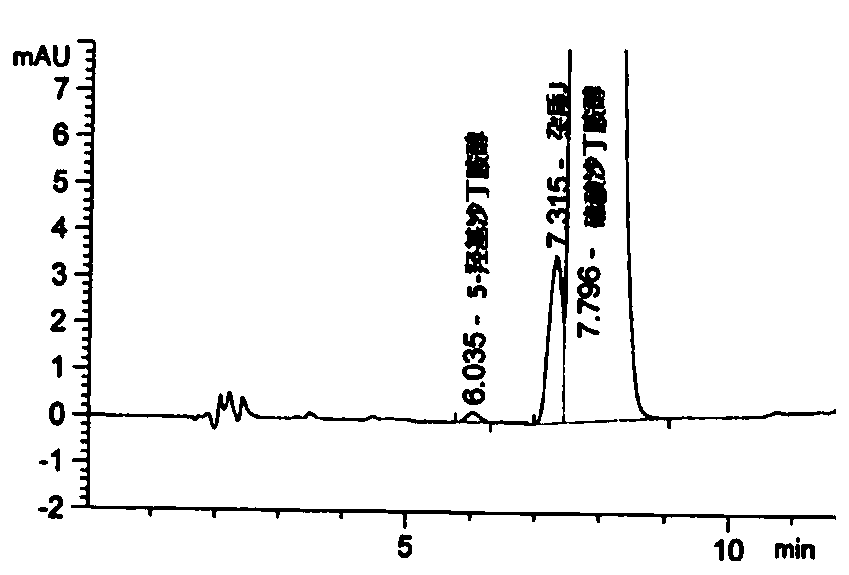
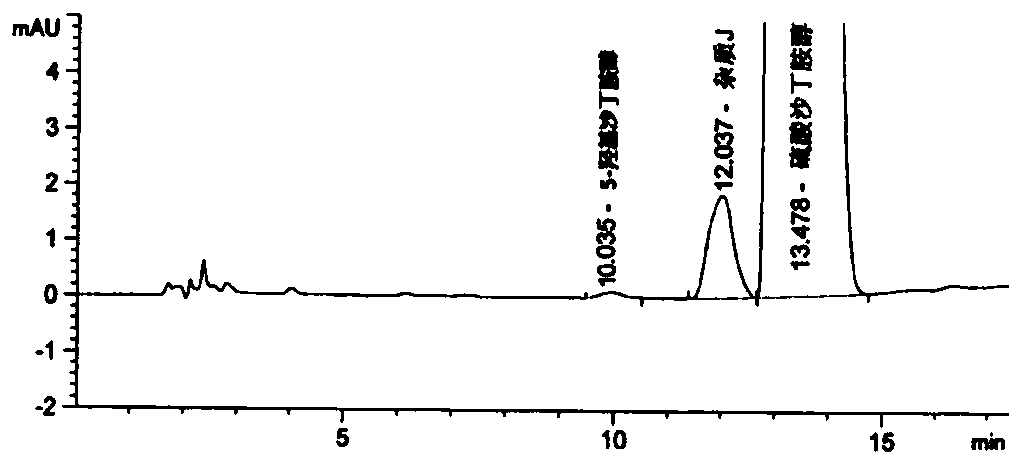
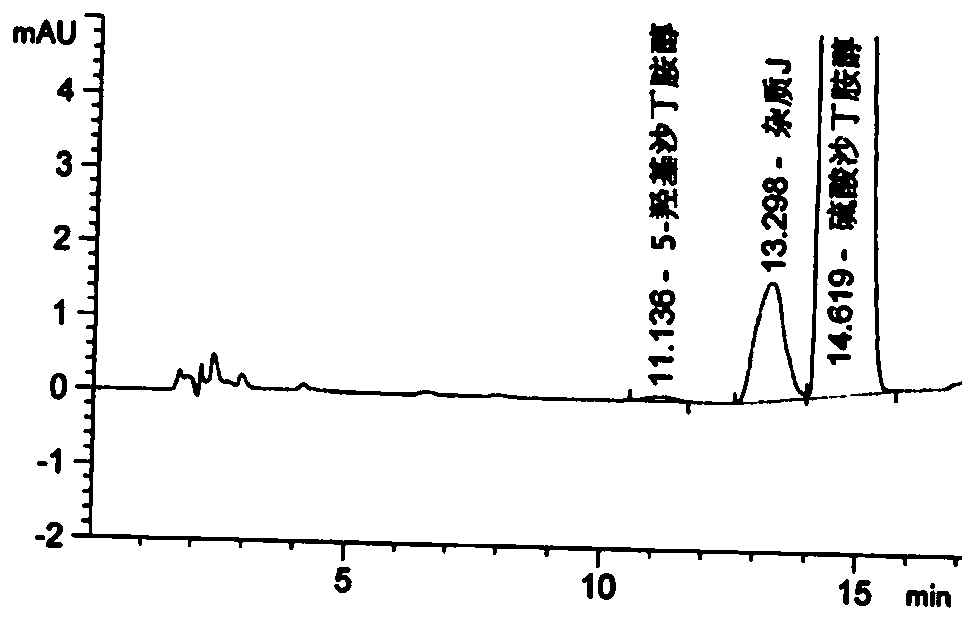
Detection method for related substances of salbutamol sulfate solution used for inhalation
InactiveCN110632205AImprove quality controlEfficient separationComponent separationPhosphateGradient elution
The invention discloses a detection method for related substances of salbutamol sulfate solution used for inhalation. A high performance liquid chromatography method is adopted for performing qualitative or / and quantitative detection on the related substances of the salbutamol sulfate solution used for inhalation, and liquid chromatography detection conditions comprise that a C8 chromatographic column is adopted, mobile phases comprise a mobile phase A and a mobile phase B, wherein the mobile phase A is phosphate buffer solution, the mobile phase B is mixed solution of acetonitrile and methanol with the volume ratio of (40-65):(60-35), and the mobile phases adopt a gradient elution method. By adopting the detection method disclosed by the invention, a main drug and other related impuritiesin the salbutamol sulfate solution used for inhalation can be effectively detetected, and the detection method has specificity and stability indication capability and also can detect a new impurity 5-hydroxy salbutamol at the same time.
Owner:SICHUAN PURITY PHARM CO LTD
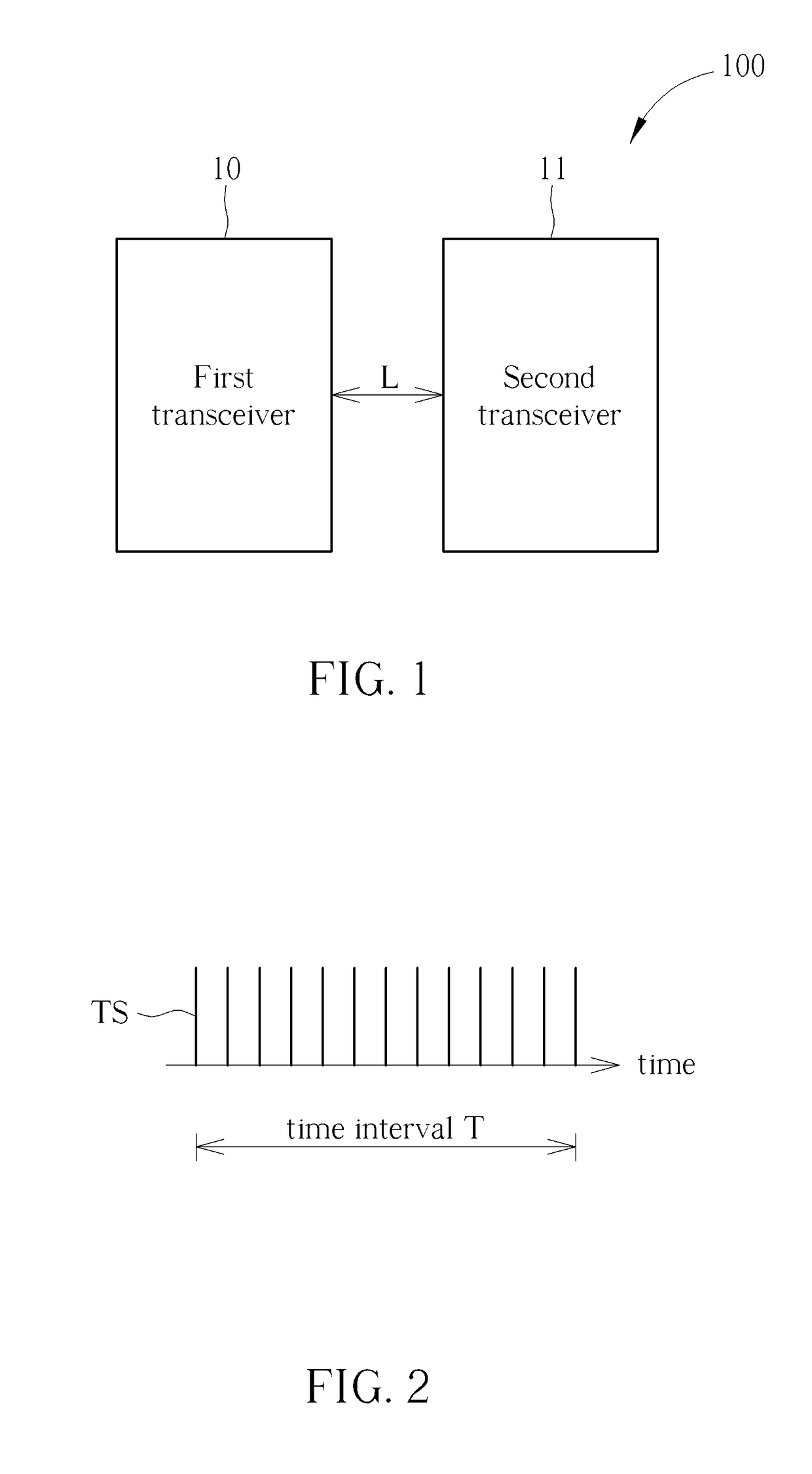
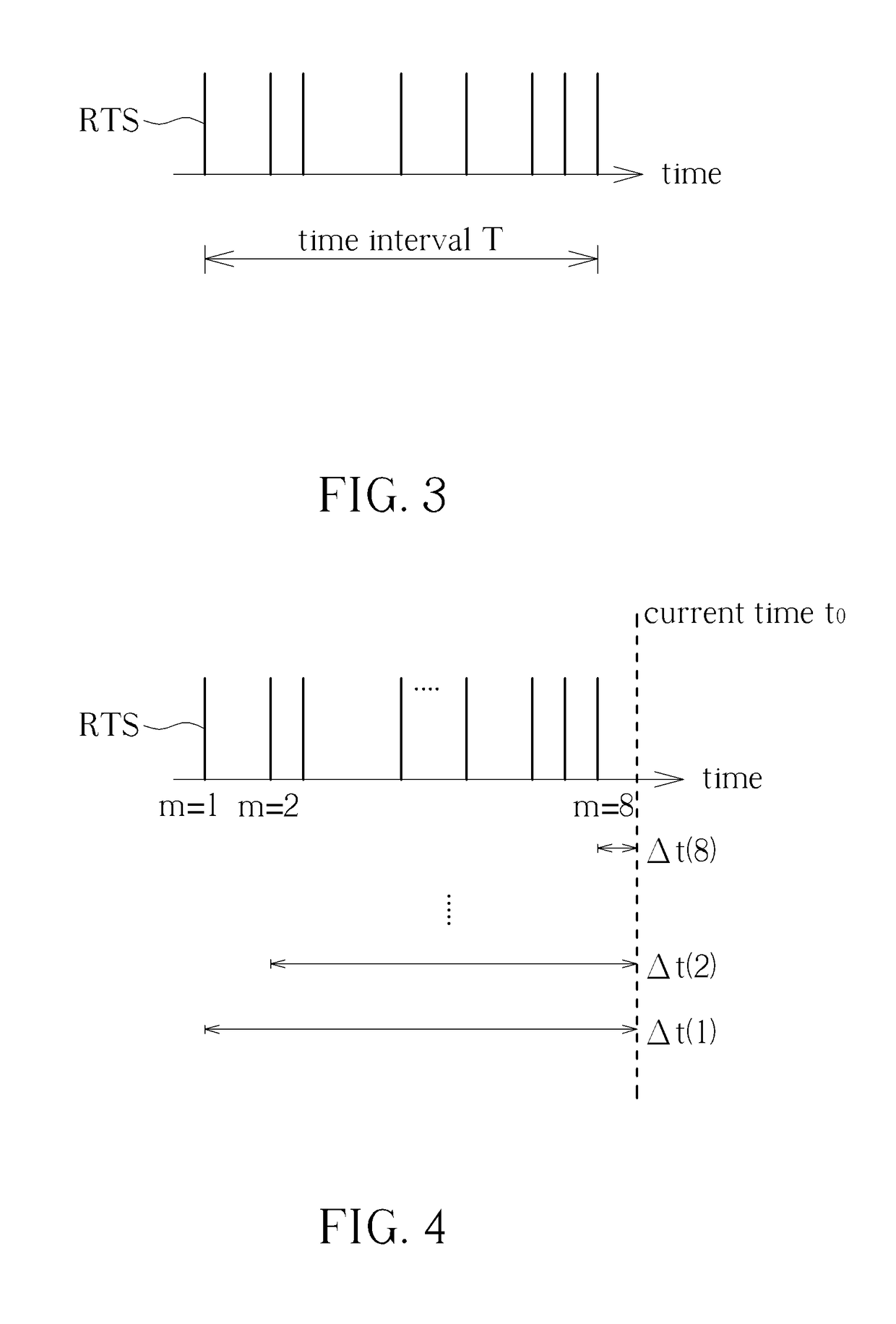
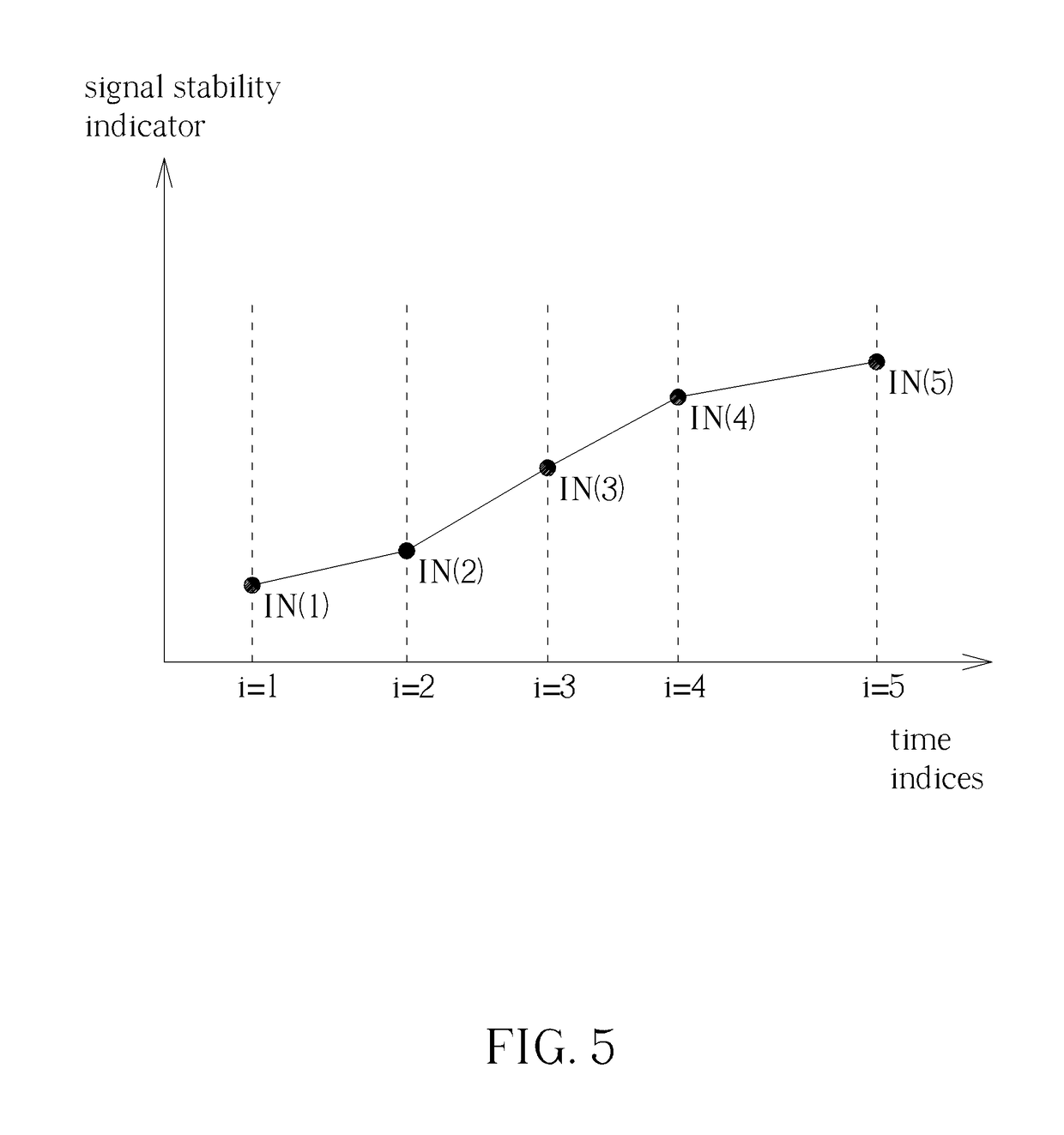
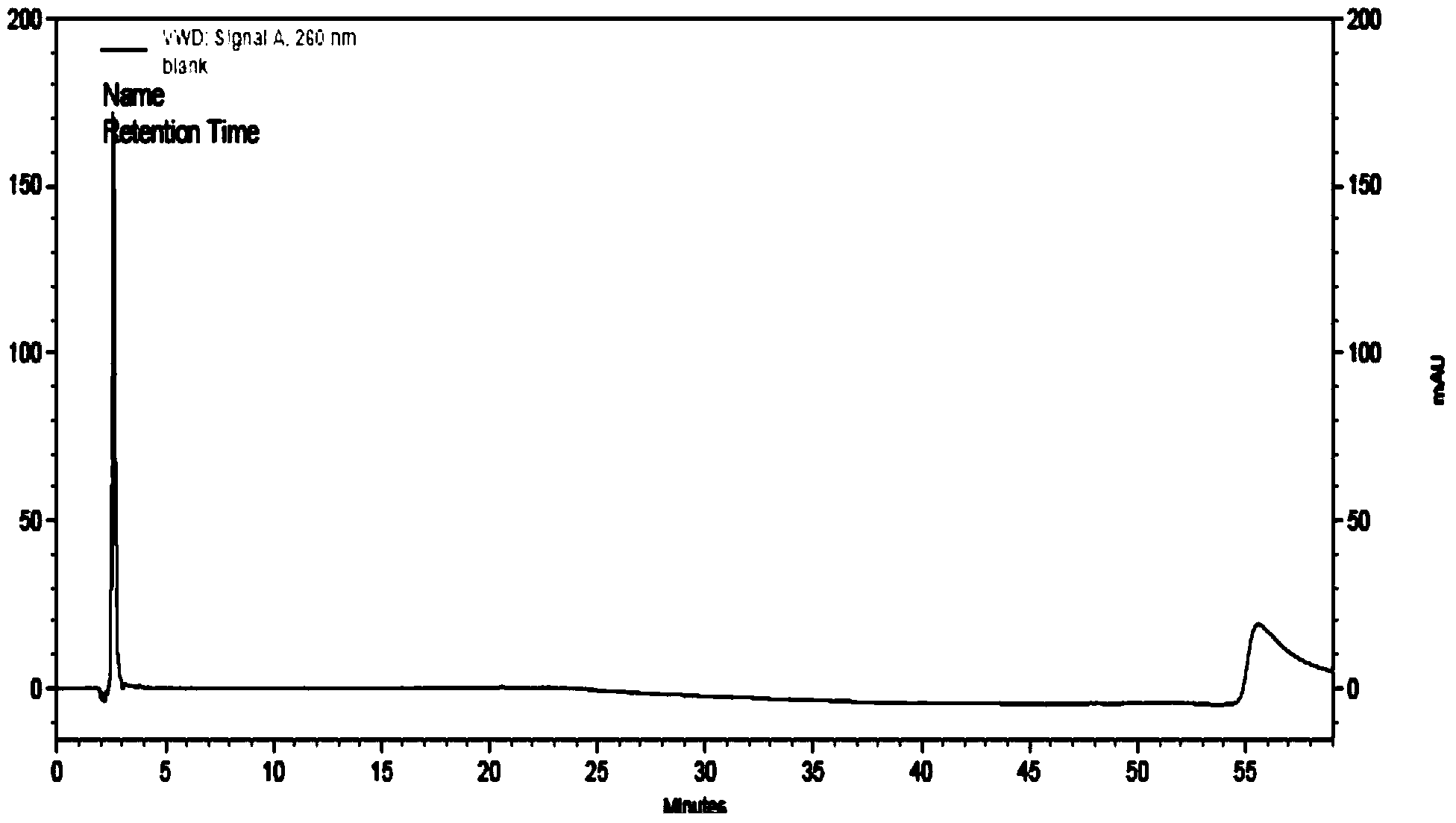
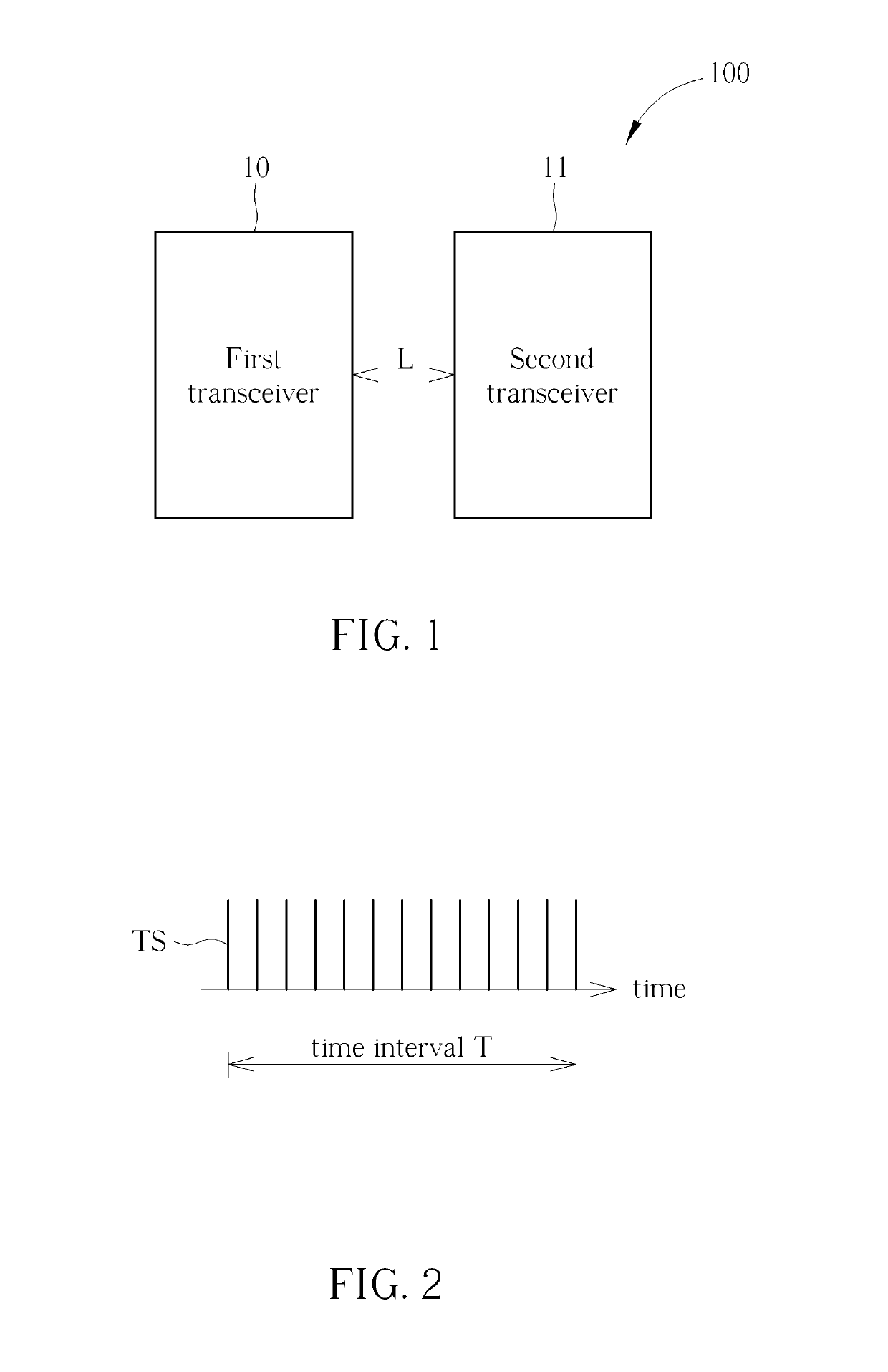
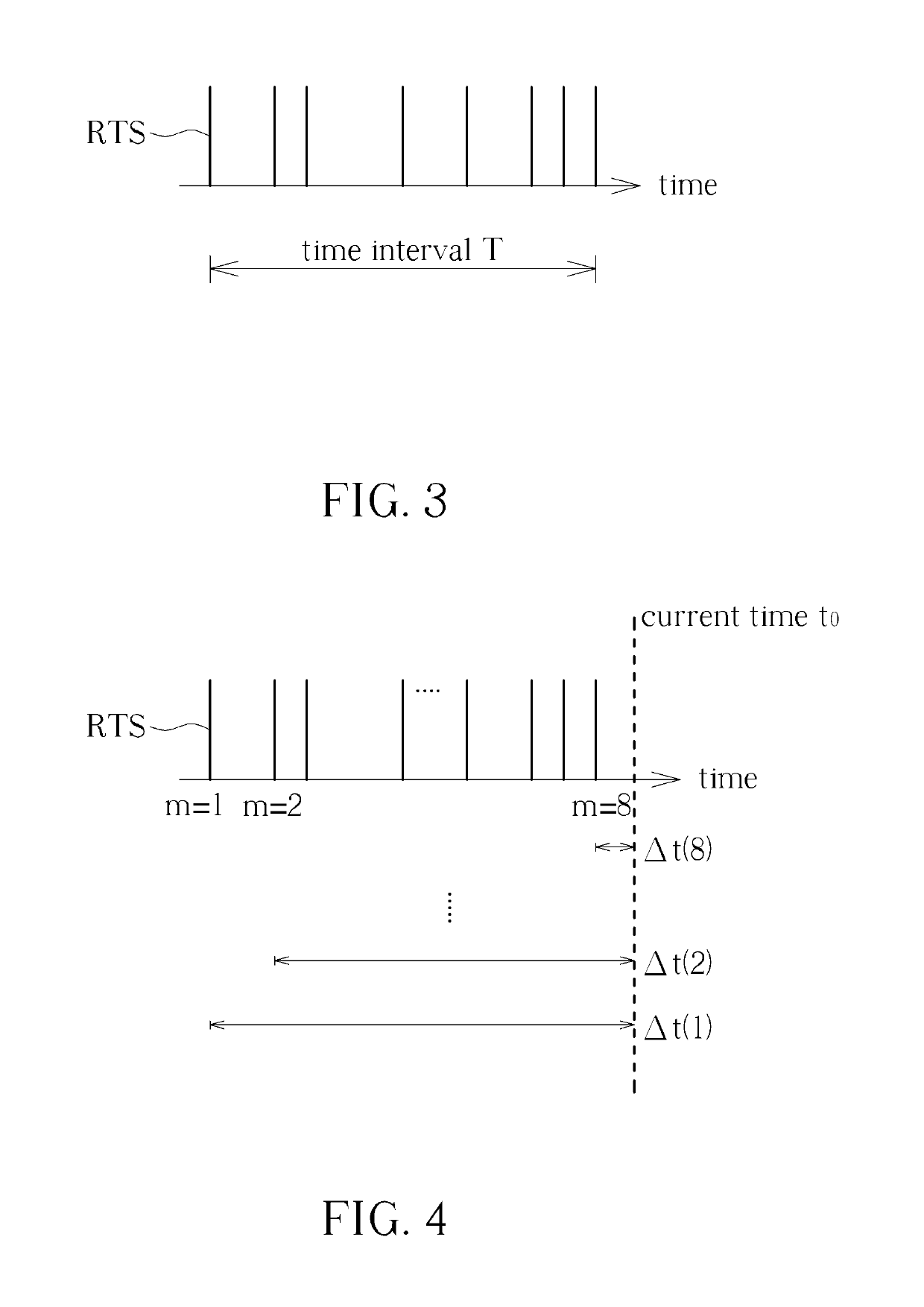
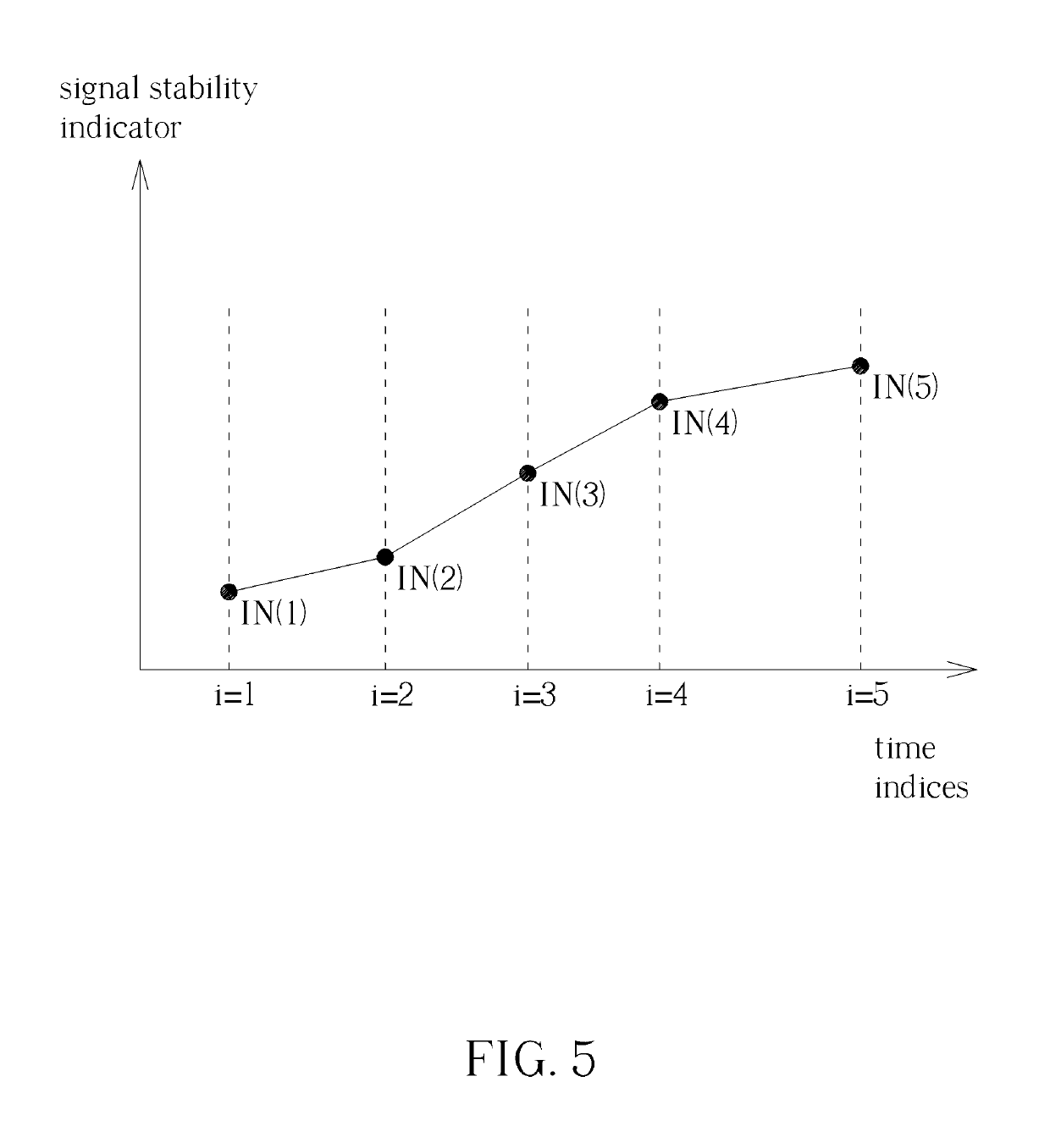
Method for Determining Stability of a Wireless Signal and System Thereof
A method for determining stability of a wireless signal includes transmitting a plurality of transmitted testing signals periodically during a time interval, receiving the plurality of transmitted testing signals and identifying at least one received testing signal from the plurality of transmitted testing signals, and generating a signal stability indicator according to a time weighting of each received testing signal of the at least one received testing signal.
Owner:QUISDA CORP
Method for separating and detecting related substances of Riociguat medicinal raw material by using HPLC (high performance liquid chromatography)
ActiveCN104374861AHigh sensitivityEfficient separationComponent separationUltraviolet detectorsStability indicating
The invention provides a method for separating and detecting related substances of a Riociguat medicinal raw material by using HPLC (high performance liquid chromatography). According to the method, an octadecylsilan bonded phase or octyl-silane bonded phase is used as a chromatographic column of filler, and an anion pair reagent solution is used as a buffer solution for eluting, and related substances in the Riociquat medicinal raw material are detected by an ultraviolet detector. The analyzing method has the advantages of good specificity, high sensitivity and good accuracy, and has relatively good stability indicating capability.
Owner:SUNSHINE LAKE PHARM CO LTD
Managing exposure to failure for computer-based systems
Methods, systems, and articles of manufacture consistent with the present invention provide for managing exposure to failure for computer-based systems. Information about a computer-based system is asynchronously received. An exposure level to failure of the computer-based system is calculated based on the received information. A stability of the computer-based system is determined based on the exposure level. A stability indication is output responsive to the determined stability.
Owner:SUN MICROSYSTEMS INC
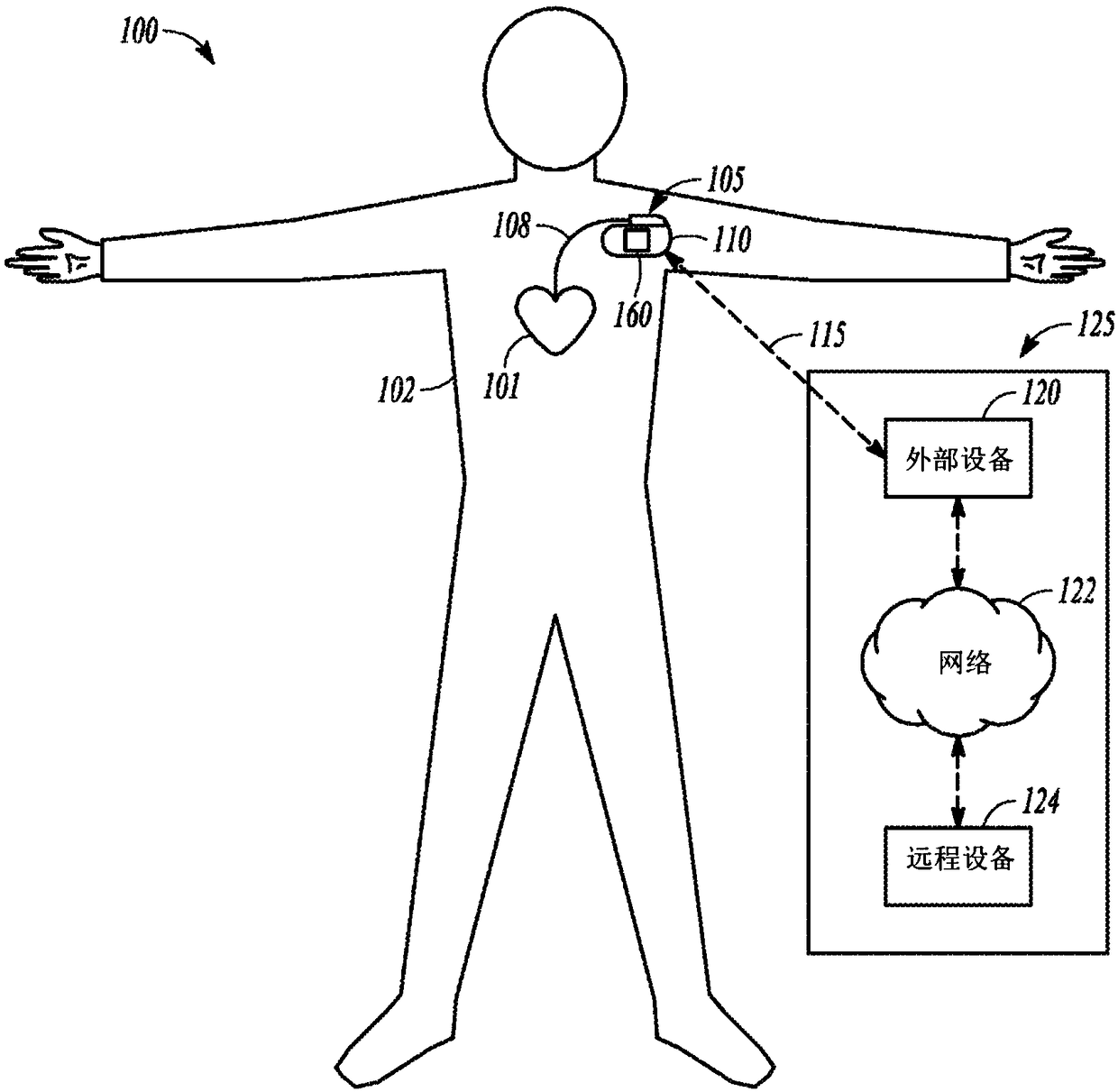
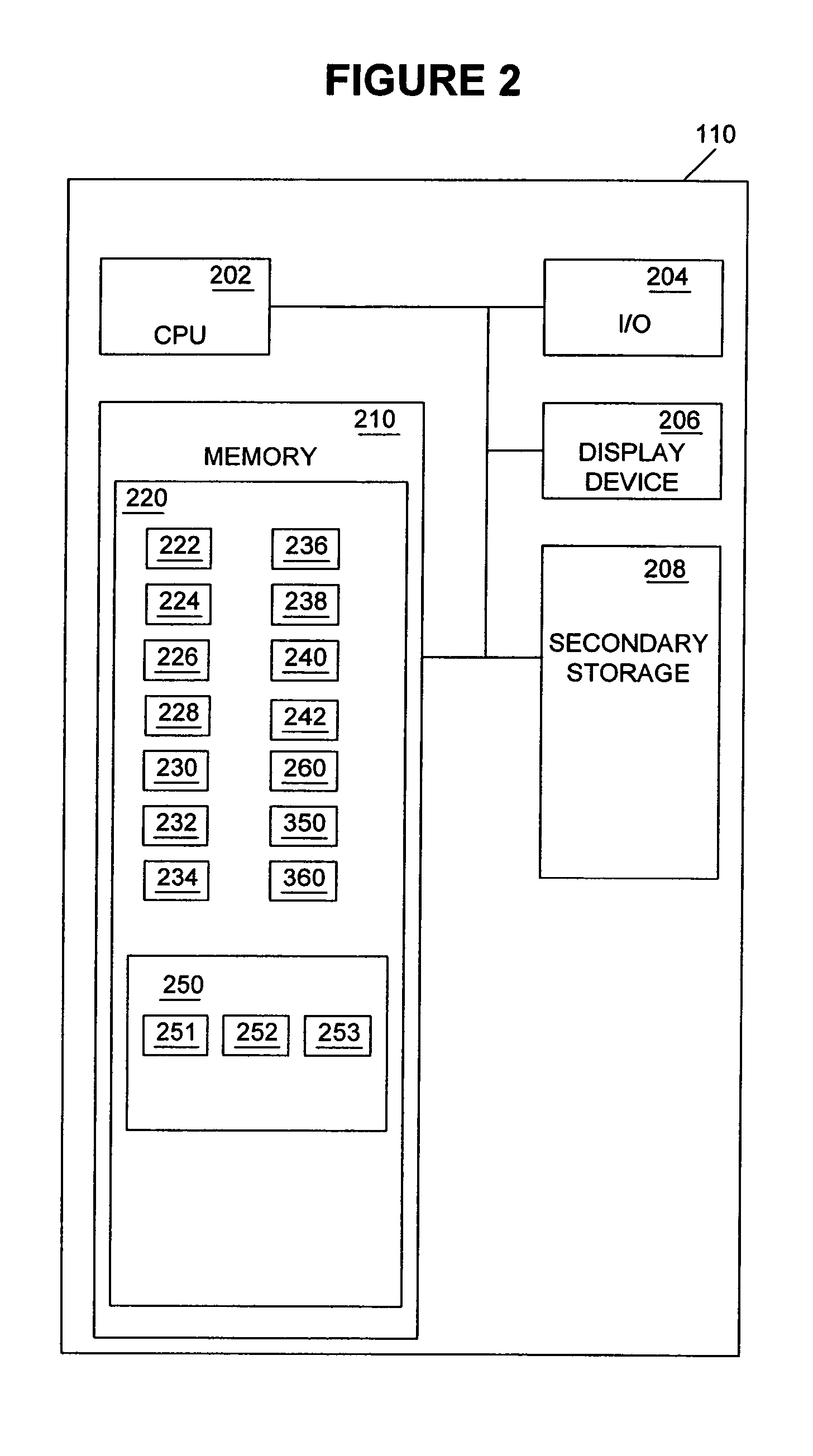
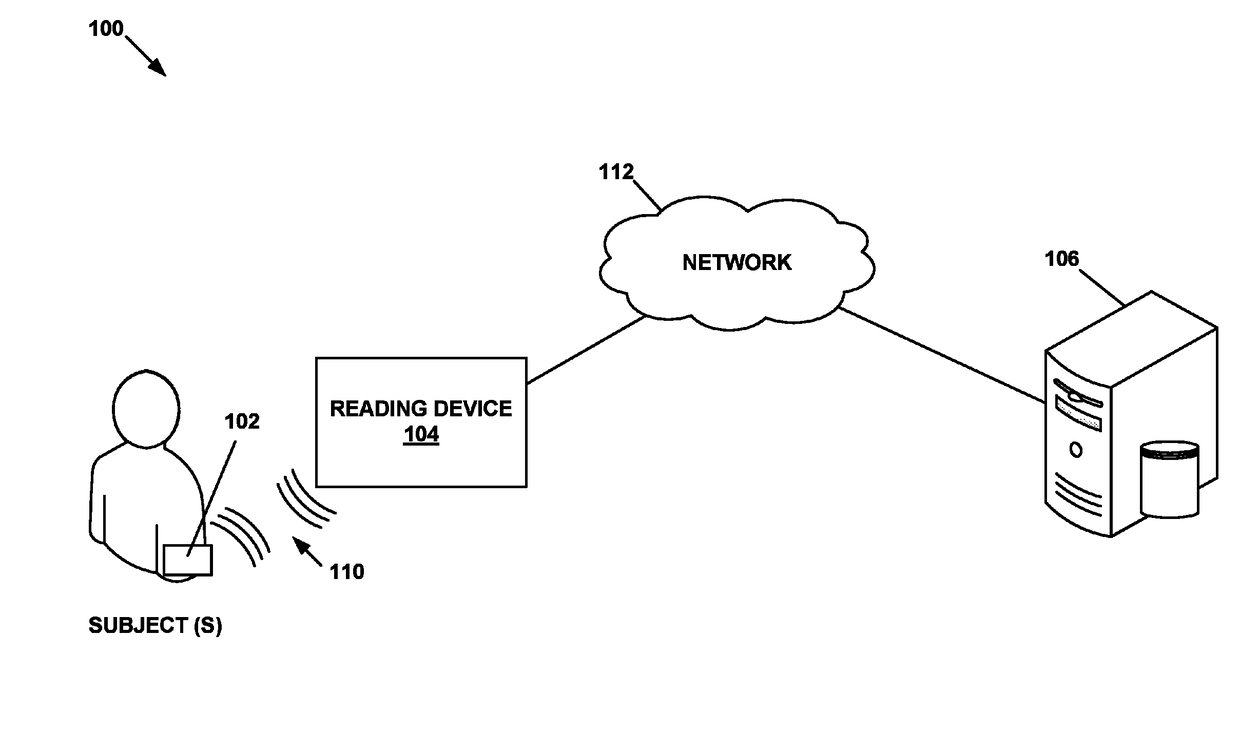
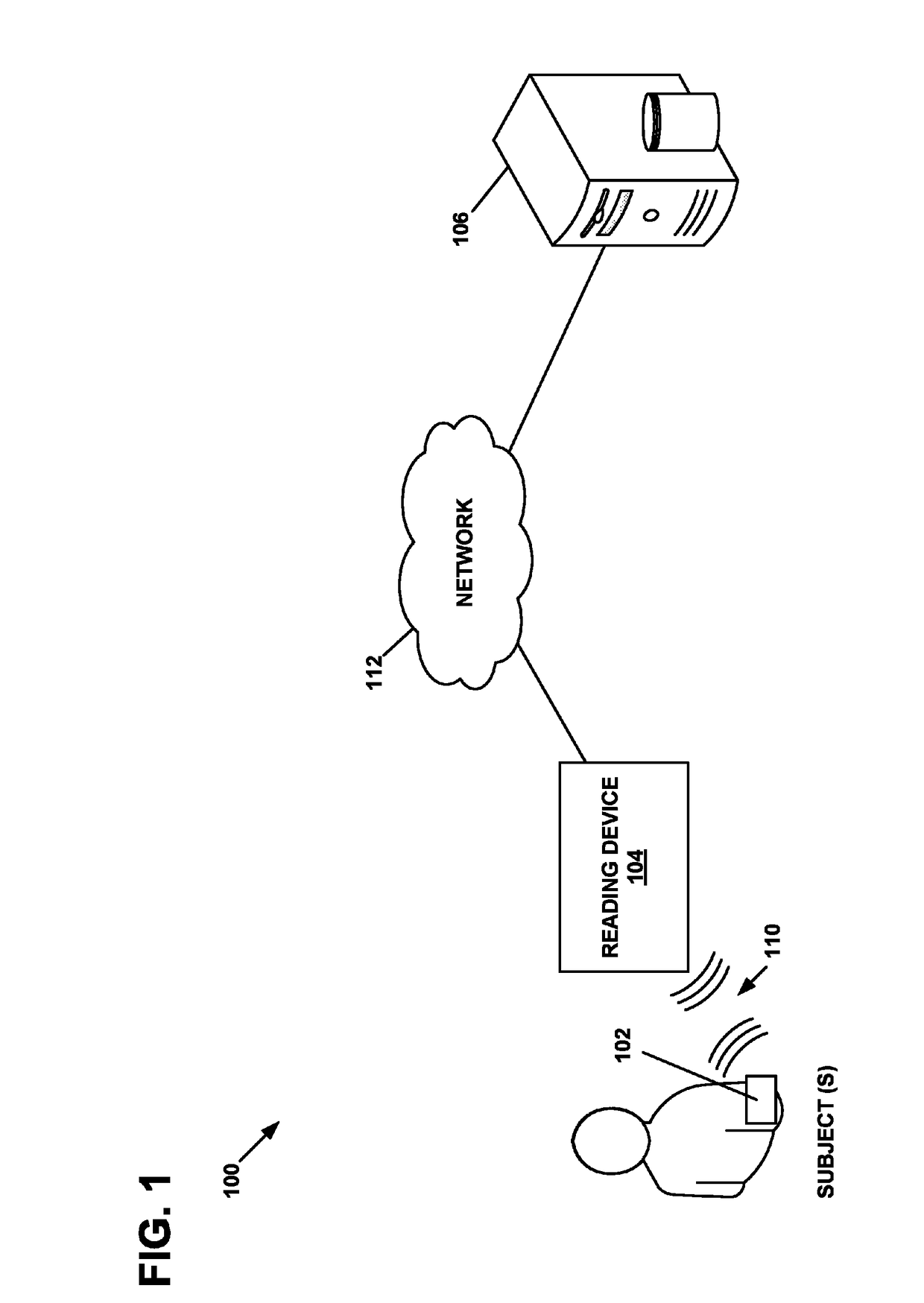
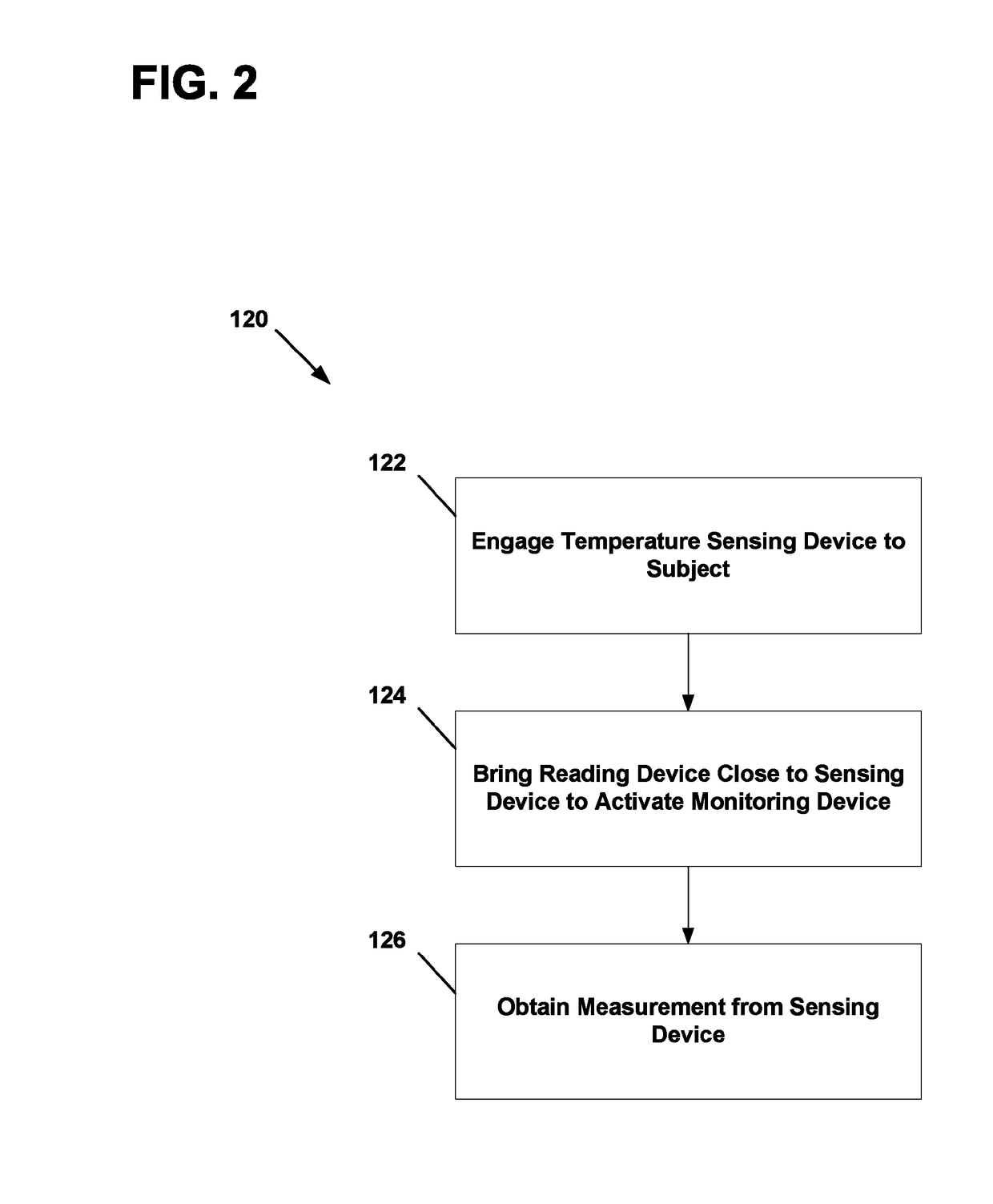
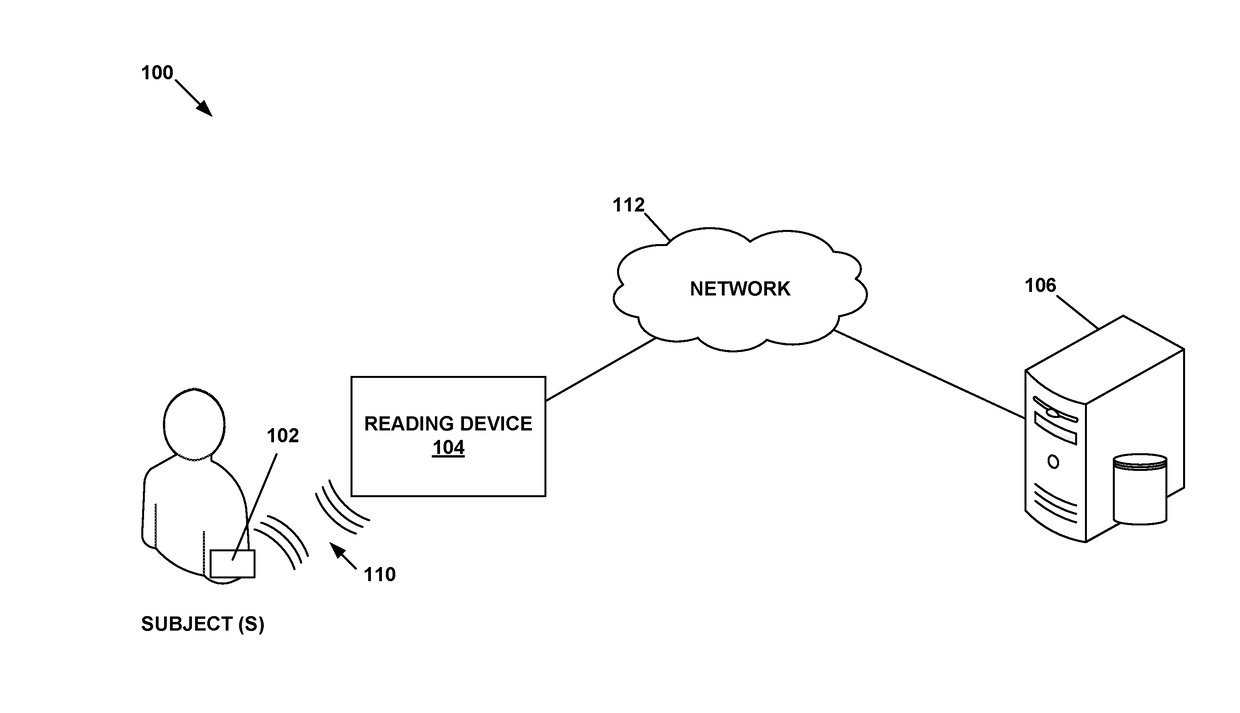
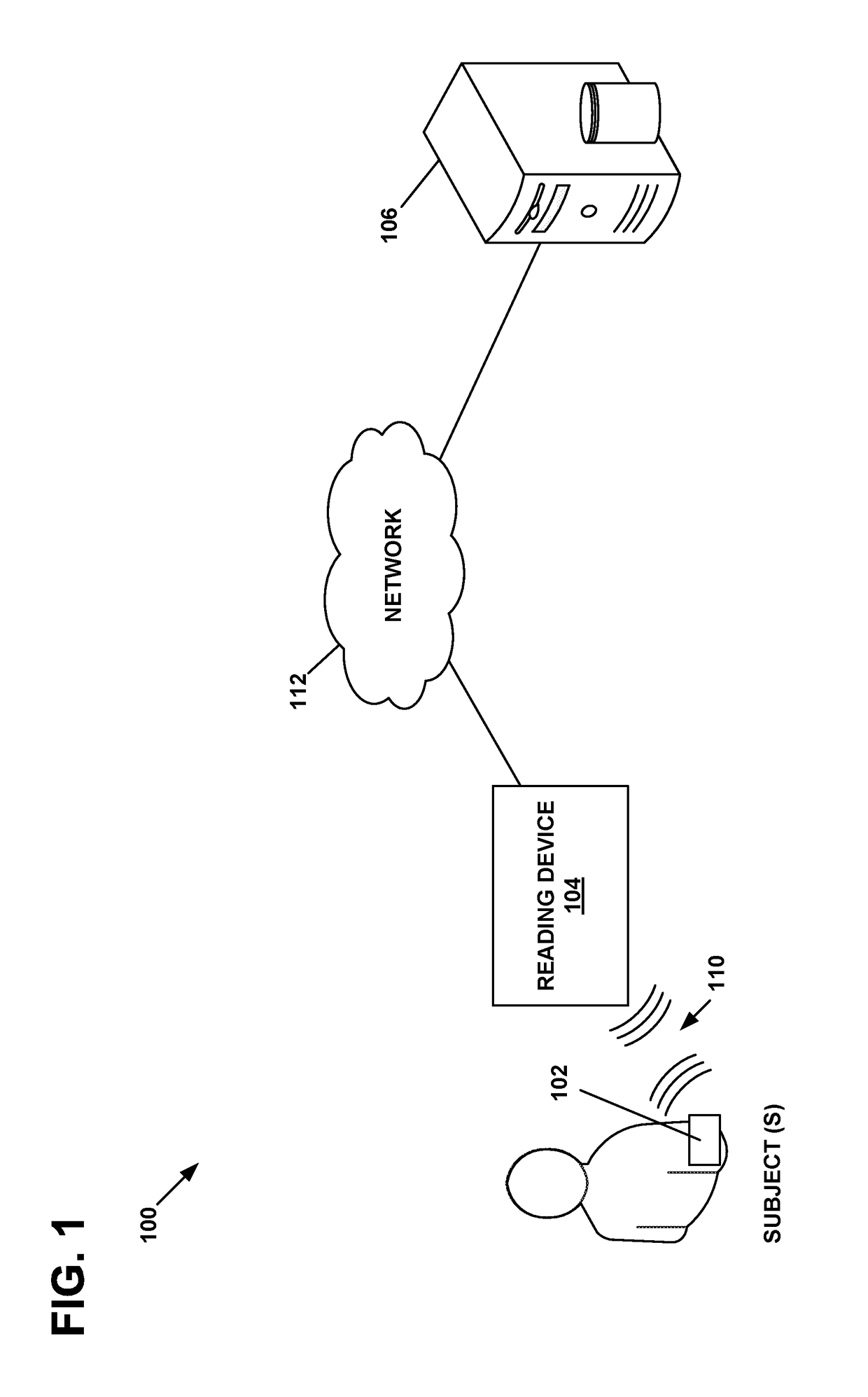
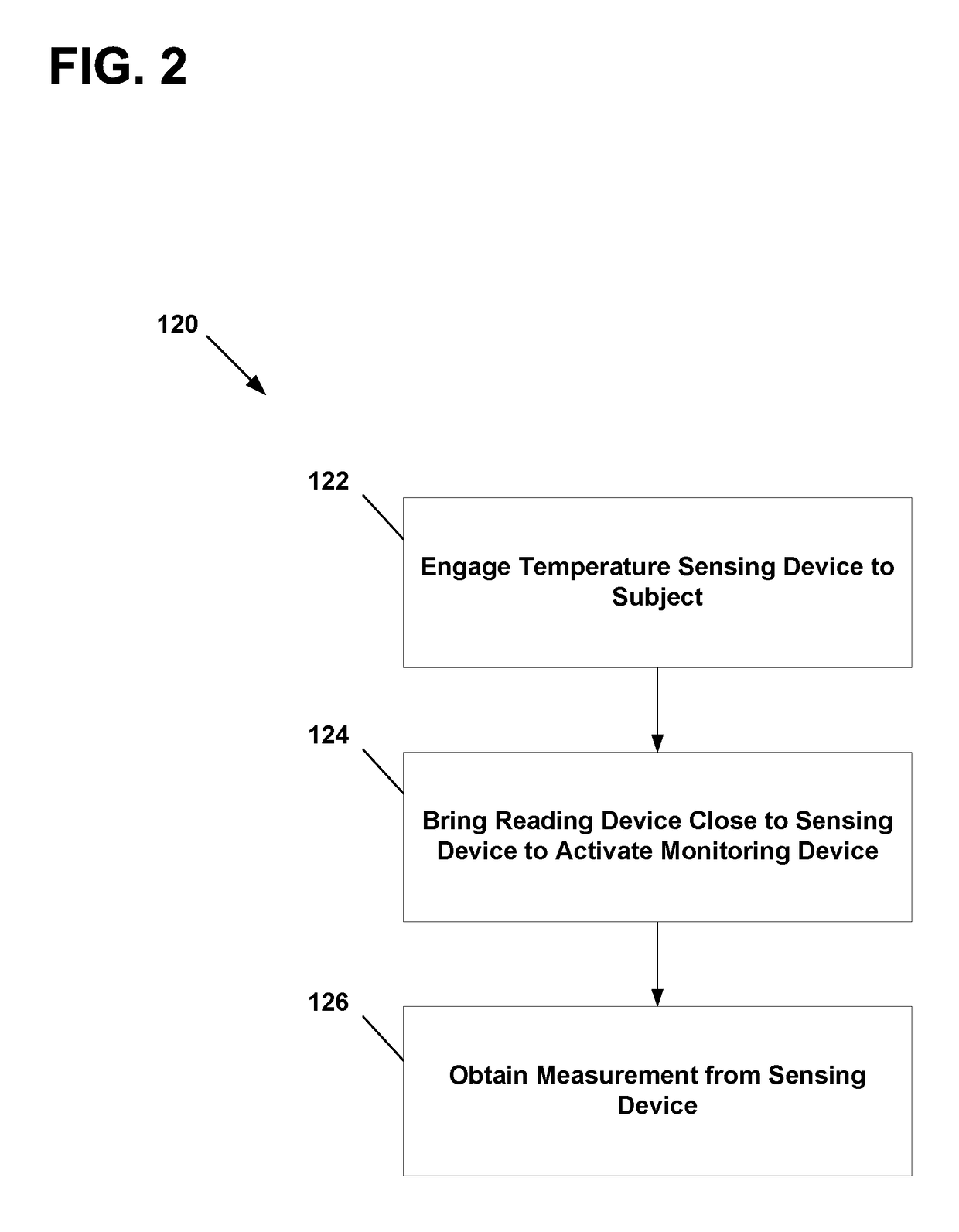
Physiological parameter measuring system
A physiological parameter measuring system includes a parameter sensing device attachable to a body of the subject. The sensing device detects at least one physiological parameter when activated, and a reading device monitors a temperature variation over time and determines whether the parameter has been so stabilized as to be reliably detected. A stability indication flag can be stored in the system so that subsequent monitoring of the parameter is instantly performed.
Owner:WELCH ALLYN INC
Physiological parameter measuring system
ActiveUS20170215729A1Electric signal transmission systemsTelemetry/telecontrol selection arrangementsStability indicatingDevice Monitor
A physiological parameter measuring system includes a parameter sensing device attachable to a body of the subject. The sensing device detects at least one physiological parameter when activated, and a reading device monitors a temperature variation over time and determines whether the parameter has been so stabilized as to be reliably detected. A stability indication flag can be stored in the system so that subsequent monitoring of the parameter is instantly performed.
Owner:WELCH ALLYN INC
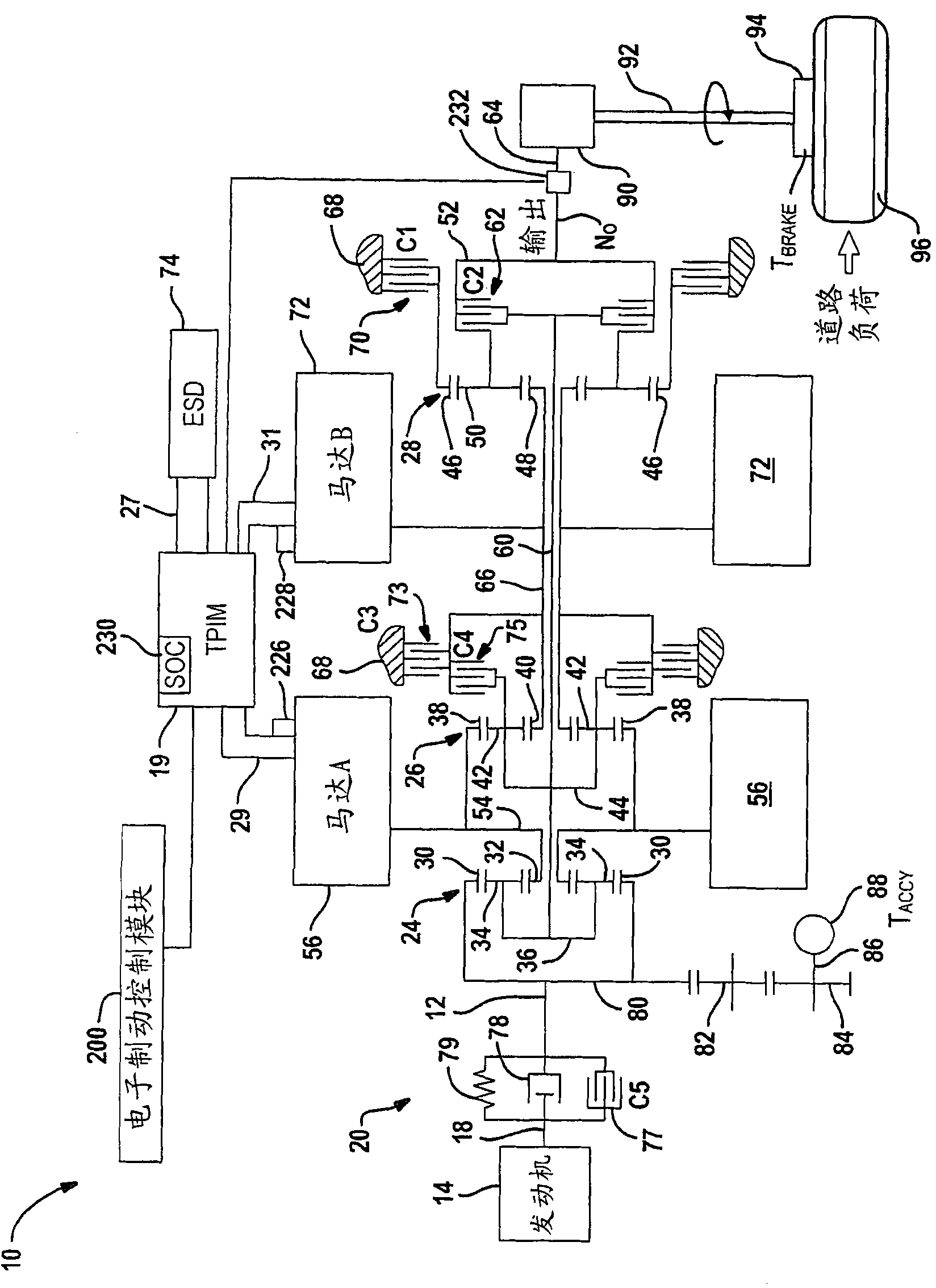
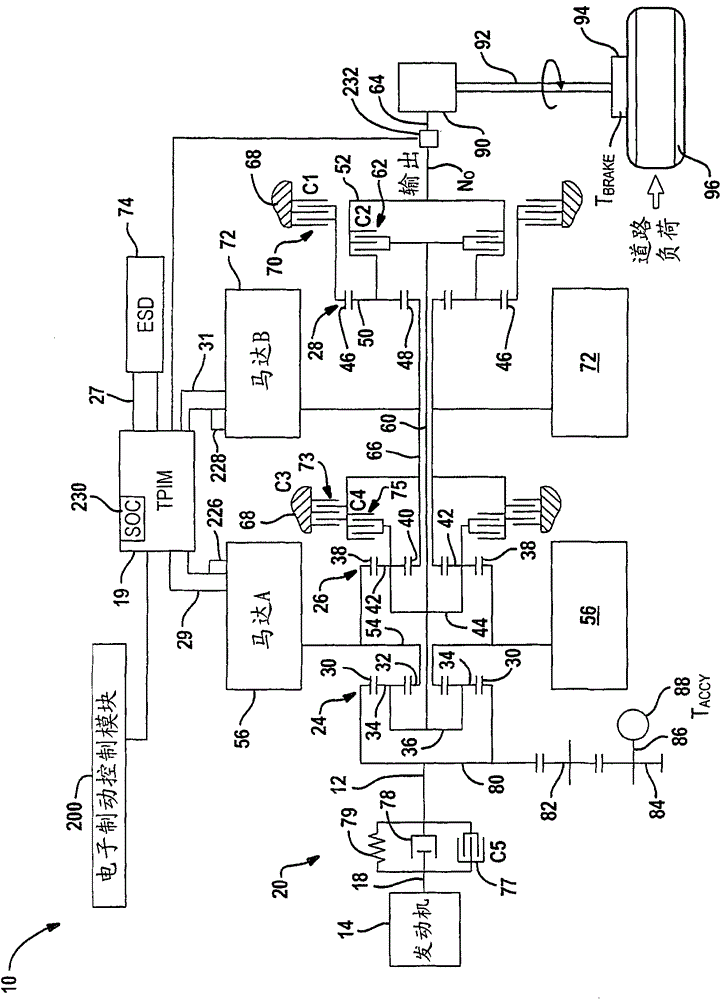
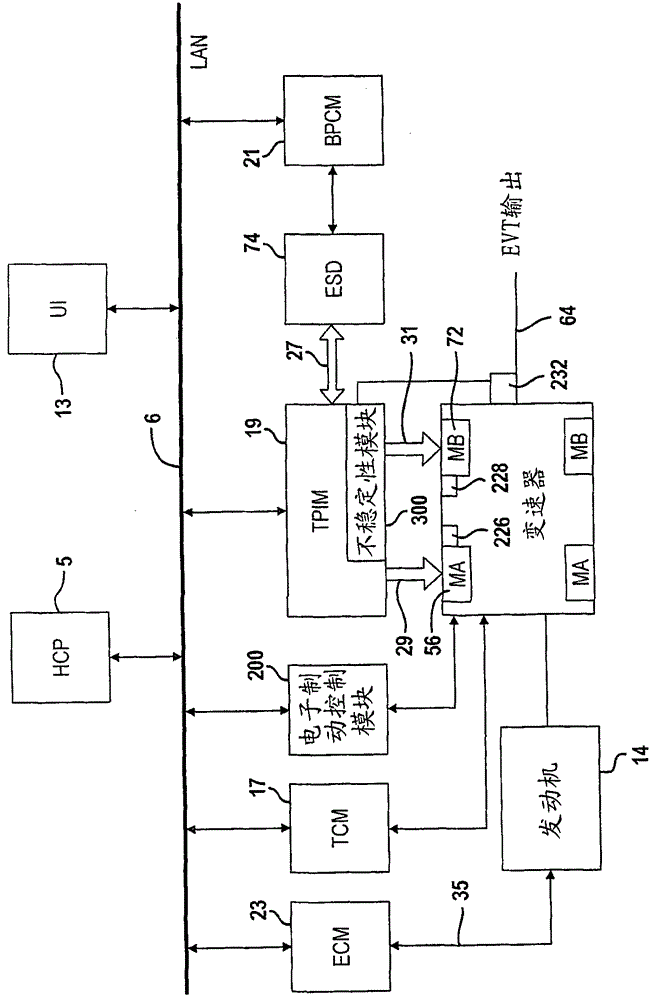
System and method for monitoring the stability of a hybrid powertrain
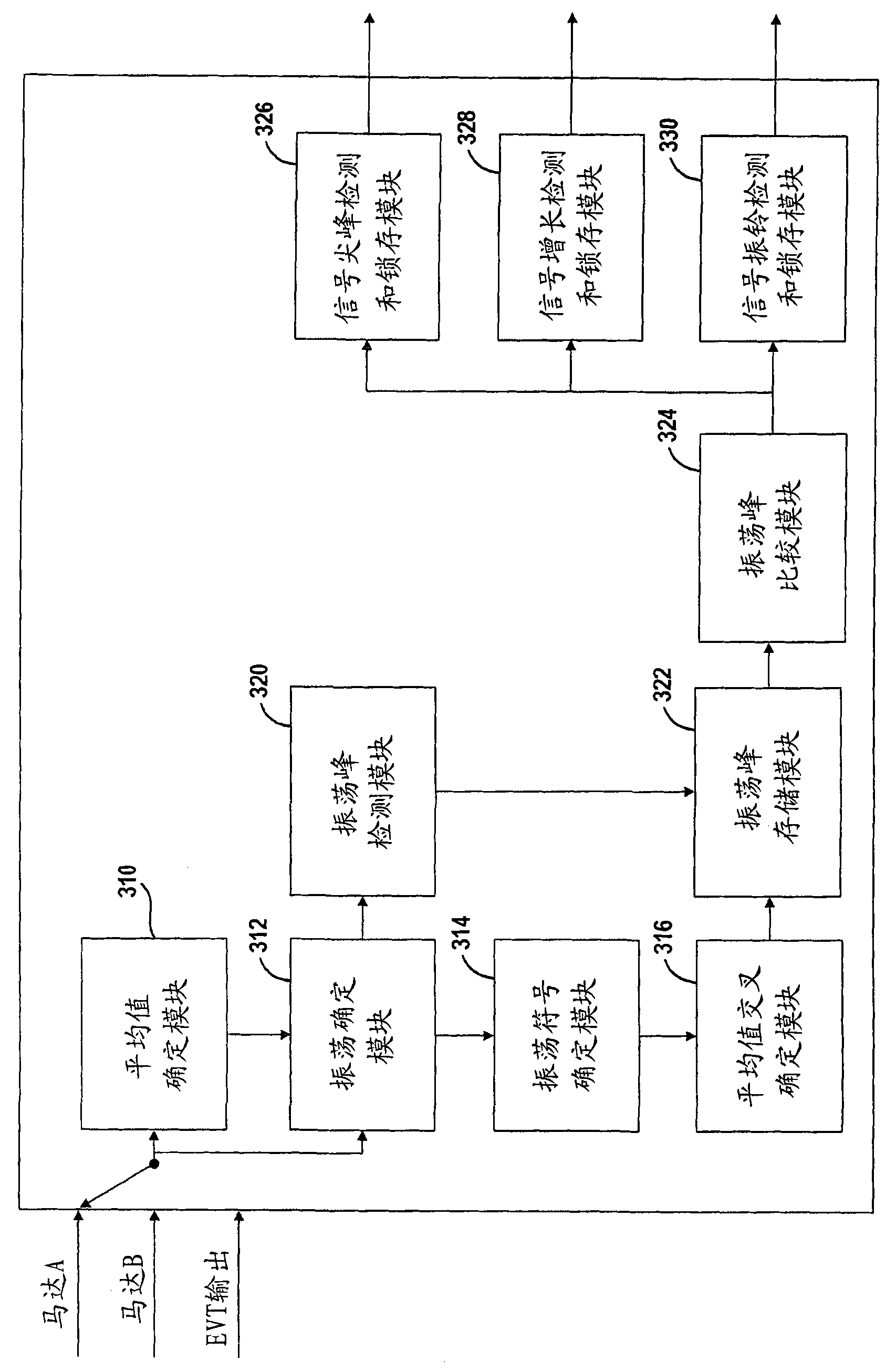
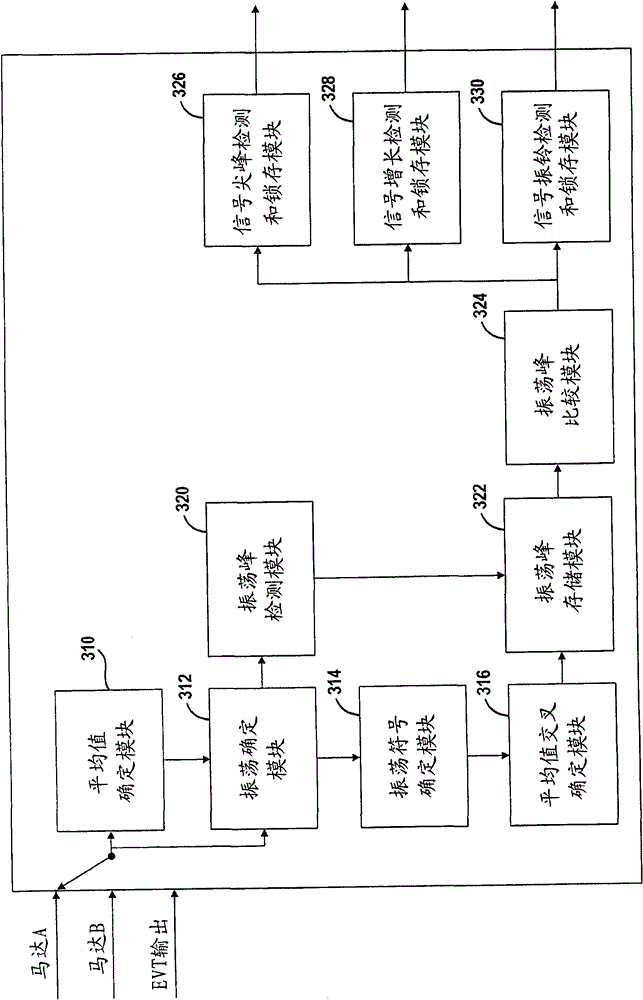
A system and method for monitoring the stability of a hybrid powertrain system includes monitoring the system and its subsystems output response signals to its inputs to identify the system operating status. For example, for a hybrid powertrain system equipped with an electrically variable transmission (EVT), three signals are monitored. They are the two electrical motor speeds representing the direct subsystem response to their feedback control inputs and the EVT output speed representing the entire system's response. A stability monitoring system includes a mean determination module that determines a mean signal of the signals and an oscillation determination module that determines a signal oscillation signal based on the instant signal and the mean signal. The monitoring system also includes a signal mean crossing determination module that determines the signal crossing its mean signal and the oscillation peak detection, storage and comparison modules that determines system instability indicators for the control system to take corrective actions.
Owner:GM GLOBAL TECH OPERATIONS LLC
A kind of assay method of related substance of methylcobalamin tablet
ActiveCN104122363BAvoid disturbing influenceImprove general performanceComponent separationPhosphateSilica gel
The invention provides a measuring method of mecobalamin tablet related substance. The measuring method comprises the steps of (1) preparing a test sample; (2) preparing reference substance; (3) measuring the content of the related substance by high performance liquid chromatography, wherein the chromatographic conditions are as follows: stationary phase is a chromatographic column taking octadecyl silane bonded silica gel as filler, mobile phase is mixed solution formed by acetonitrile and phosphate buffer solution, the detection wave length is 300-360nm, the sample amount is 20mu l, the column temperature is 30 DEG C, and the flow velocity is 0.5-1.5ml / min; gradient elution is carried out; and (4) calculating the content of all impurities in the test sample. The measuring method of the mecobalamin tablet related substance is high in universality, good in reproducibility, high in degree of separation and accurate in detection result; the methodology validation proves that the measuring method has a stable indicative function, and is capable of rapidly and accurately controlling the limit of all the impurities in mecobalamin tablets, so that the quality can be controlled; furthermore, the measuring method is low in cost, and has good economic benefit and popularization prospect.
Owner:HANGZHOU CONBA PHARMA
Method for detecting (R)-etiracetam from medicine
The invention discloses a method for detecting (R)-etiracetam (R-isomer) from levetiracetam injection. Qualitative and / or quantitative detection is carried out on the R-isomer through adoption of a high performance liquid chromatography. Liquid chromatography detection conditions comprise the fact that a chromatographic column is a cellulose-tris(3, 5-dichlorophenyl carbamate) silica gel column; and a mobile phase comprises n-hexane and anhydrous ethanol, wherein n-hexane (vol.%): anhydrous ethanol (vol.%) is equal to 90-70: 10-30, and isocratic elution is employed for the mobile phase. Through adoption of the method provided by the invention, the (R)-etiracetam in the levetiracetam injection can be effectively detected, and the method has specificity and stability indication capacity. Thelevetiracetam injection can be directly detected after being diluted. A sample does not need to be preprocessed. The method is convenient and rapid. According to the method provided by the invention,limit of detection reaches 0.003%, limit of quantitation reaches 0.005%, and the method is accurate and sensitive.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
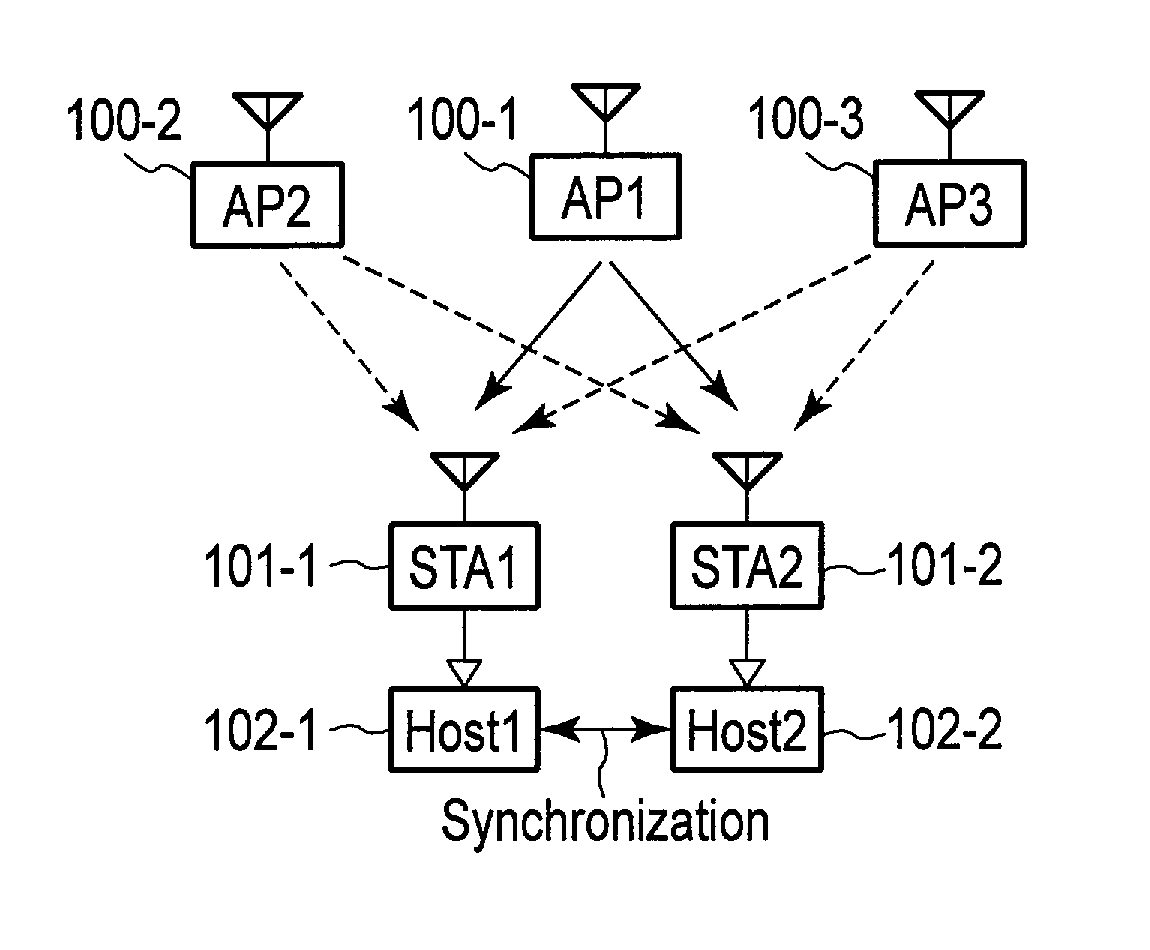
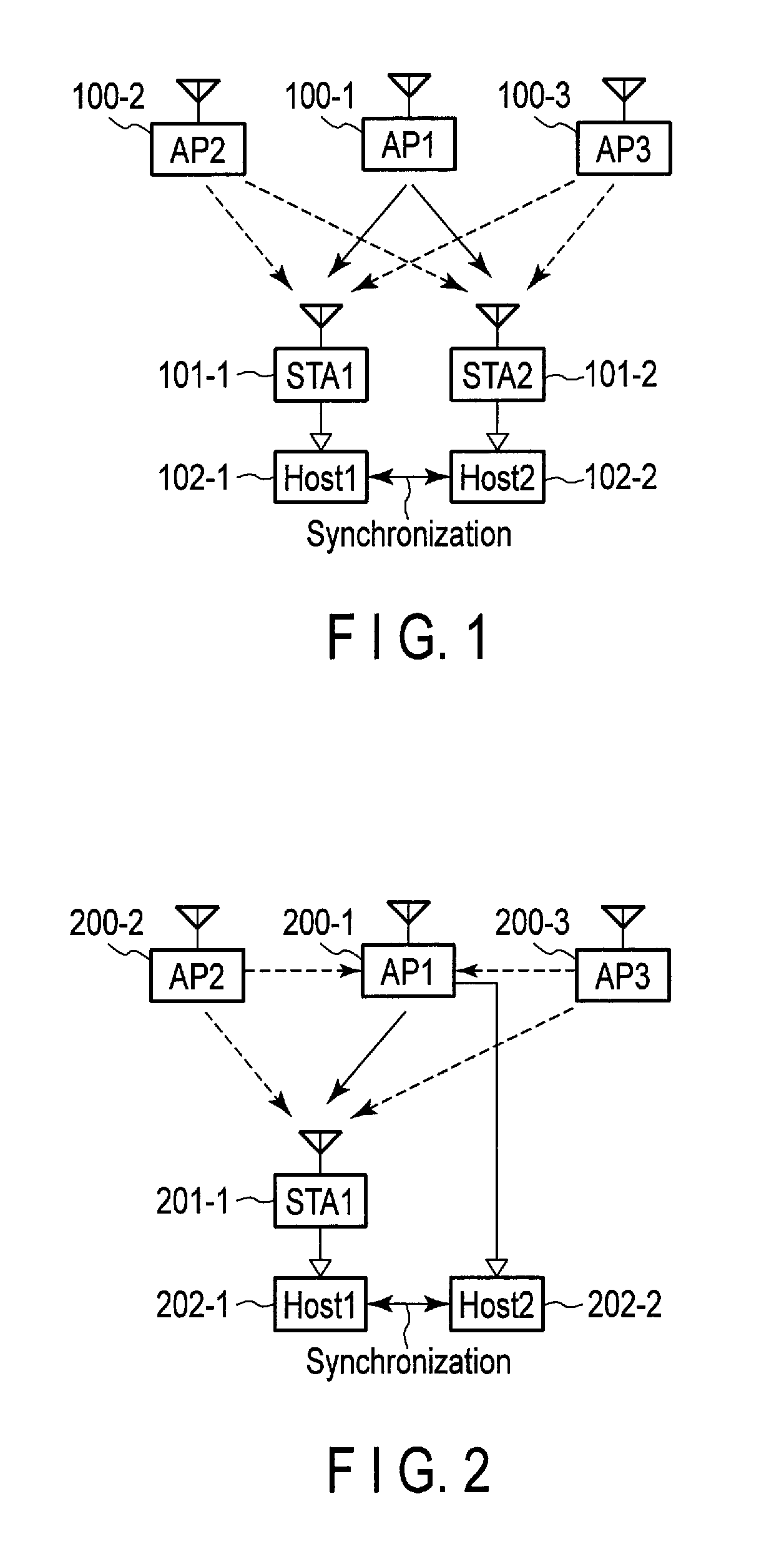
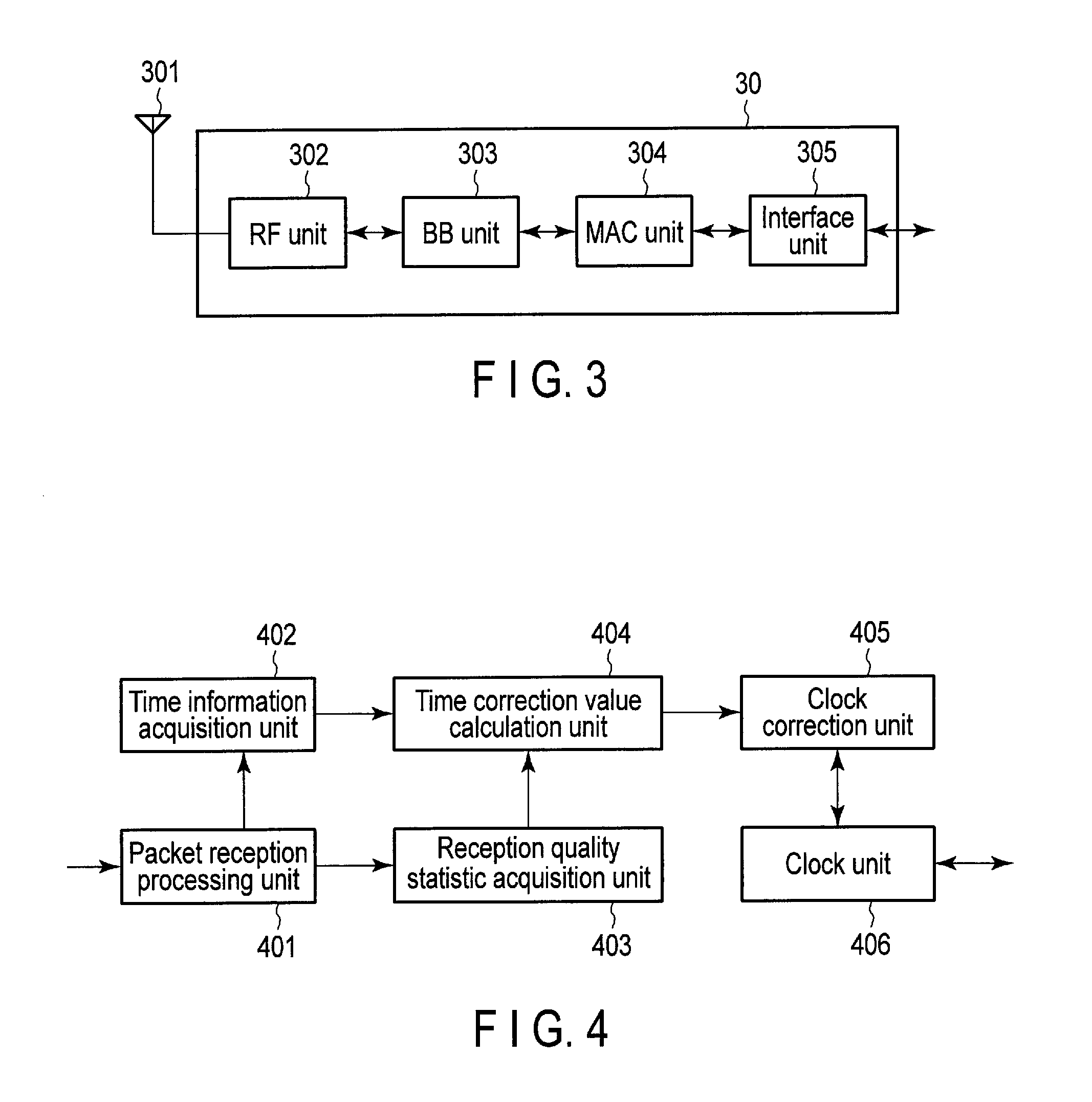
Wireless communication apparatus and wireless communication system
According to an embodiment, a wireless communication unit includes a media access control (MAC) unit. The MAC unit includes a clock unit, an acquisition unit and a calculation unit. The clock unit includes a register which stores periodically counted-up first time information. The acquisition unit acquires a statistic for reception quality information on the received packet. The calculation unit normalizes the second time information, weights the normalized second time information using a time weight based on the statistic, and calculates a time correction value for correcting the first time information, using the weighted and normalized second time information. The time weight increases with increasing link stability indicated by the statistic.
Owner:KK TOSHIBA
A method for detecting Devetiracetam from medicine
ActiveCN109765316BQuality improvementMeet impurity control requirementsComponent separationCelluloseCarbamate
The invention discloses a method for detecting devetiracetam (R-isomer) from levetiracetam injection, and adopts high performance liquid chromatography to carry out qualitative or / and quantitative detection of the R-isomer, The detection condition of liquid chromatography comprises: chromatographic column: cellulose-three (3,5-dichlorophenyl carbamate) silica gel column; Mobile phase: n-hexane, dehydrated alcohol; Wherein, n-hexane (vol.% ): absolute ethanol (vol.%)=90~70: 10~30; the mobile phase adopts isocratic elution. The method of the invention can effectively detect the levetiracetam in the levetiracetam injection, and has specificity and stability indicating ability. Moreover, the levetiracetam injection can be directly diluted and tested without pretreatment of samples, which is convenient and fast; the detection limit of the method of the invention reaches 0.003%, and the quantification limit reaches 0.005%, which is accurate and sensitive.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
A method for detecting 4-(1-(2,5-dimethylphenyl)ethyl)-1h-imidazole or/and its hydrochloride
ActiveCN108872431BDetect without interferenceMaintain stabilityComponent separationEthyl groupP phosphate
The invention discloses a method for detecting 4-(1-(2,5-dimethyl phenyl)ethyl)-1H-imidazole or / and a hydrochloride thereof from medetomidine or dexmedetomidine hydroch; HPLC (High Performance LiquidChromatography) method is used for qualitative or / and quantitative detection of the 4-(1-(2,5-dimethyl phenyl)ethyl)-1H-imidazole or / and the hydrochloride thereof, and detection conditions of LC (Liquid Chromatogram) include: chromatographic column: C18 chromatographic column, mobile phase includes an aqueous phase and an organic phase, wherein the aqueous phase is a phosphate buffer; the organicphase is a methanol or / and acetonitrile solution, and the organic phase is selected from methanol or / and acetonitrile; the mobile phase is eluted by isocratic elution method; the method of the invention can effectively detect 4-(1-(2,5-dimethyl phenyl)ethyl)-1H-imidazole or / and the hydrochloride thereof in the bulk pharmaceutical chemical, capable of indicating specificity and stability.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Image processing device and display control method
Owner:SONY CORP
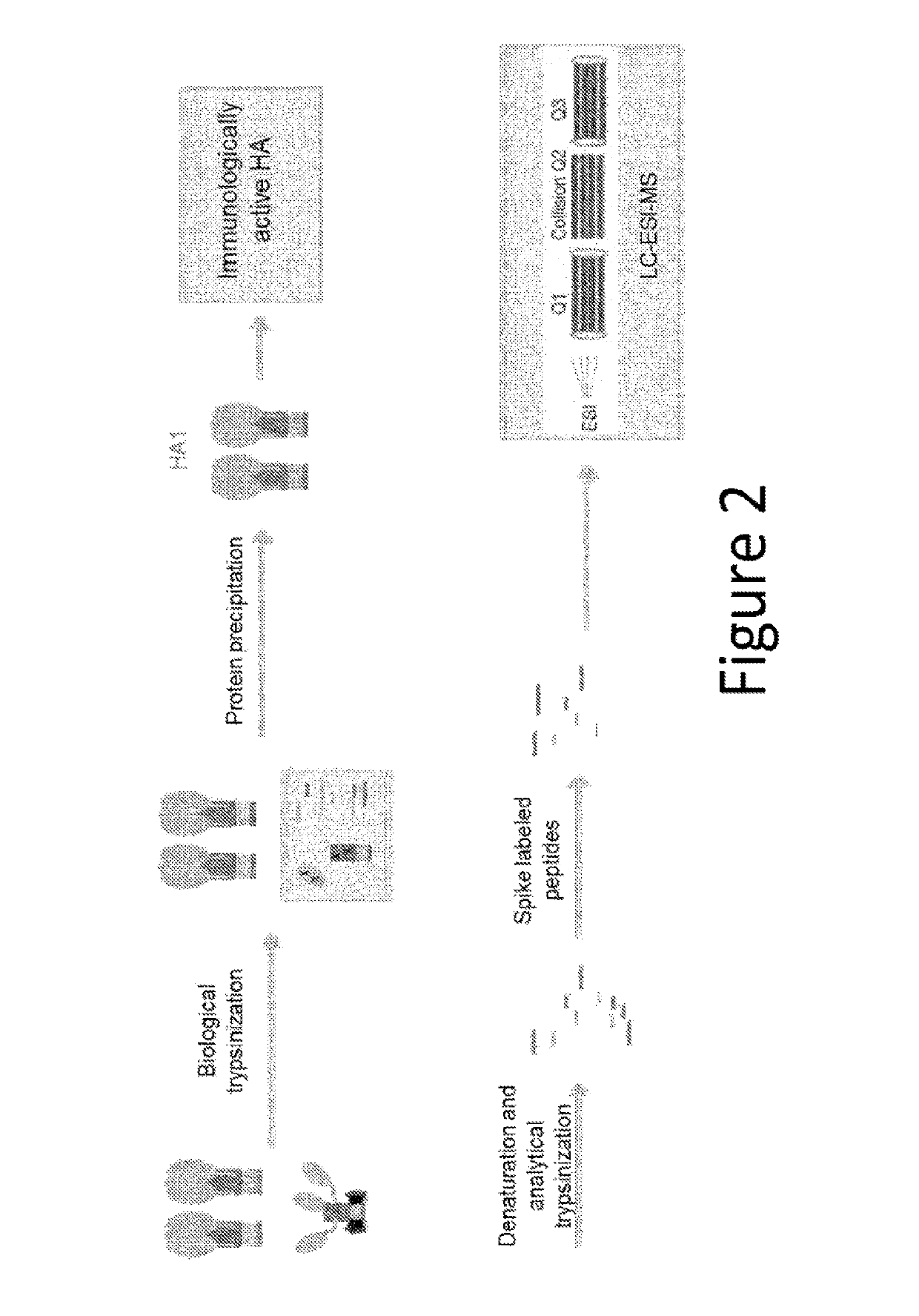
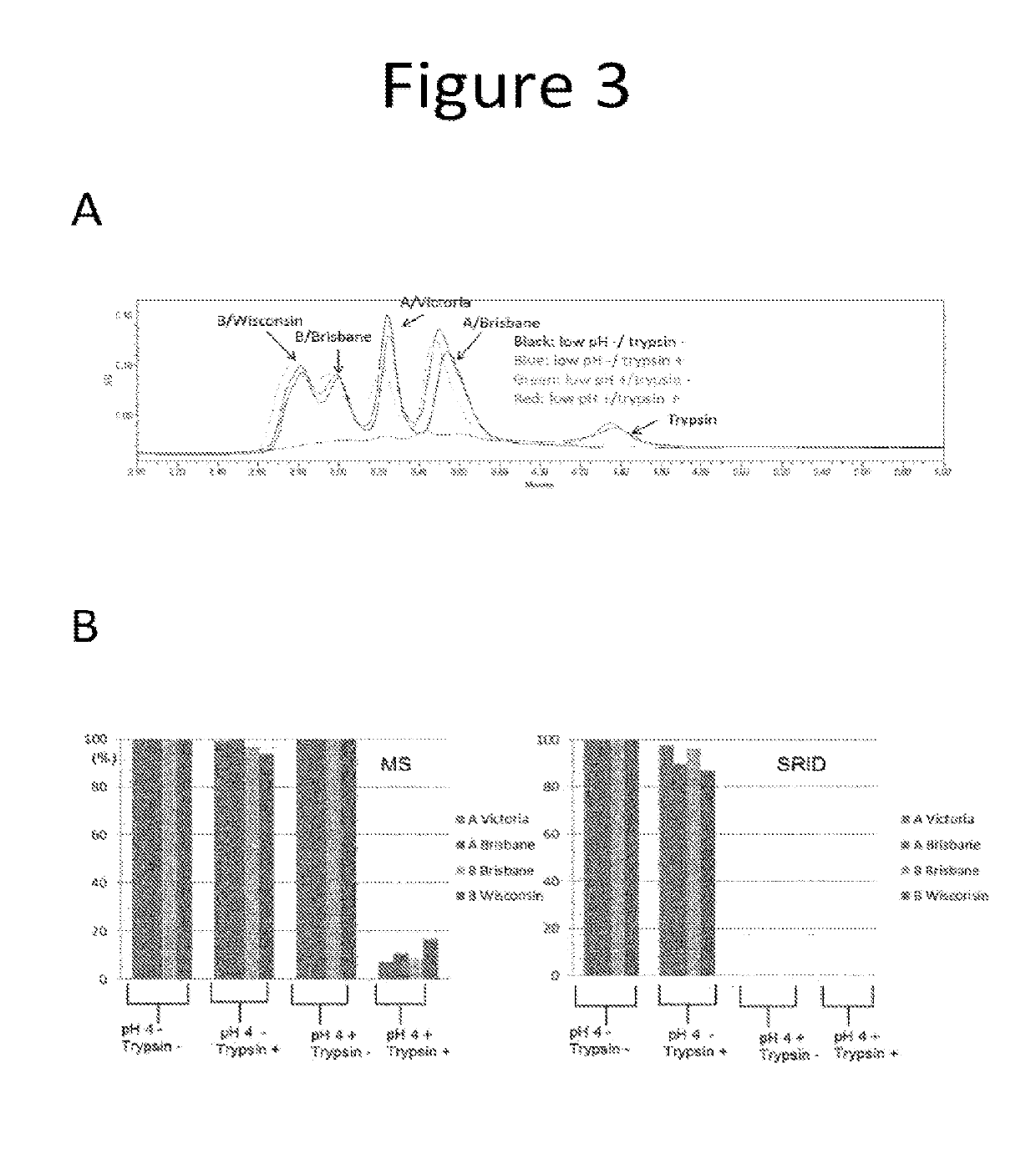
Influenza potency assays
ActiveUS10416171B2Accurate measurementThe right amountSsRNA viruses negative-senseComponent separationStability indicatingInfluenza vaccine
Owner:SEQIRUS UK LTD
Stability indicator
InactiveCN1515681ASlow down the rate of formationLong shelf lifeMicrobiological testing/measurementBiological testingHigh concentrationPeroxidase
The present invention relates to an indicator of a kind of bio-chemical reagents which uses peroxidase as indication system. By 1000 ml of reagent the composition of said indicator contains MOPSO 7.21g, MOPSO sodium salt 4.45g, sodium azide 0.2-1.0g, bovine serum albumin 0.2g, Qulatong-1002 5g, 4-aminoantipyrene 1.0-3.0g, peroxidase 10-30 ku and phenol-formic acid, 1.0-4.0g. By adding high-concentration sodium azide as [O] removing agent the formation speed of red quinoinmine chromopexis can be greatly delayed, the storage time of the reagent can be greatly prolonged. After one year of storage at 2-8 deg.C, the blank absorbancy of the reagent is still below 0.2A, its stabilizing time is above one year.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
Method for determining stability of a wireless signal and system thereof
A method for determining stability of a wireless signal includes transmitting a plurality of transmitted testing signals periodically during a time interval, receiving the plurality of transmitted testing signals and identifying at least one received testing signal from the plurality of transmitted testing signals, and generating a signal stability indicator according to a time weighting of each received testing signal of the at least one received testing signal.
Owner:QISDA CORP
A method for detecting related substances in piperacillin sodium and sulbactam sodium for injection
The invention provides a method for detecting related substances in piperacillin sodium and sulbactum sodium for injection. The method adopts an octadecyl silane bonded silica-gel chromatographic column to carry out gradient elution so as to rapidly and precisely complete the detection on related substances in piperacillin sodium and sulbactum sodium. In the obtained chromatogram, the main related substance (2S)-2-amino-3-methyl-3-sulfinobutyric acid and piperacillin penicilloic acid are well separated, piperacillin and sulbactum are also well separated from other related substances, and the separation degree is more than 1.5. The degradation researches and methodology on piperacillin sodium and sulbactum sodium show that the provided method has a stability indicating function, and thus the detection method can be used to control the limits of impurities in piperacillin sodium and sulbactum sodium, and can also be used to control the quality of piperacillin sodium and sulbactum sodium for injection. The method has the advantages of convenient operation and low cost, and has a good economic profit and promotion prospect.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
System and method for monitoring the stability of a hybrid powertrain
The present invention relates to systems and methods for monitoring the stability of a hybrid powertrain. A system and method for monitoring stability of a hybrid powertrain system includes monitoring output response signals of the system and its subsystems to their inputs to identify system operating conditions. For example, for a hybrid powertrain system equipped with an electrically variable transmission (EVT), three signals are monitored. These are the two motor speeds representing the immediate subsystem response to its feedback control input and the EVT output speed representing the overall system response. A stability monitoring system includes: an average value determination module, which determines an average value signal of the signal; and an oscillation determination module, which determines the signal oscillation signal according to the instantaneous signal and the average value signal. The monitoring system also includes: a signal mean value crossing determination module for determining a signal crossing with its mean value signal; and an oscillation peak detection, storage and comparison module for determining a system instability indicator for causing the control system to take corrective action.
Owner:GM GLOBAL TECH OPERATIONS LLC
Stability indicator for a wireless repeater
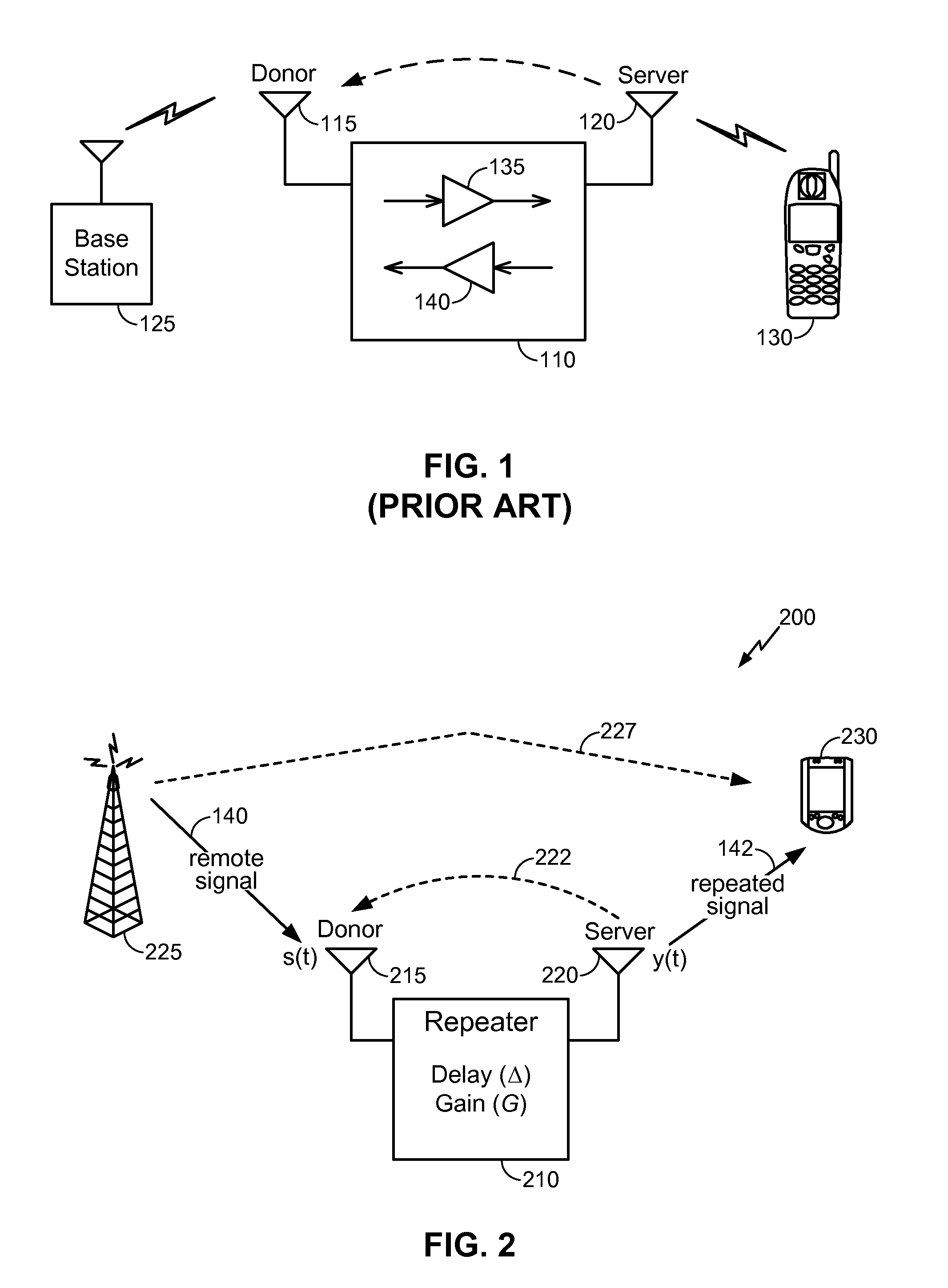
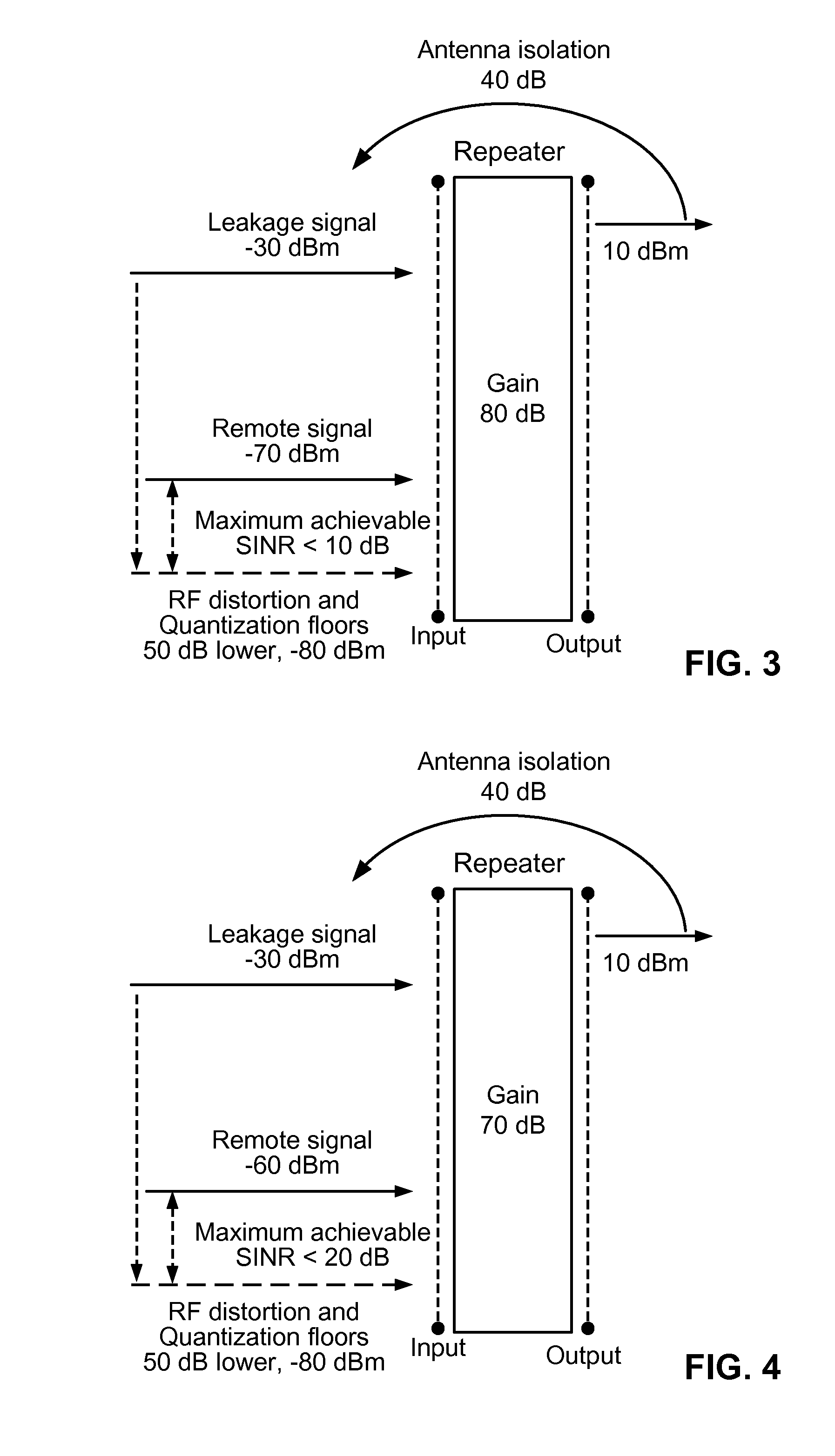
InactiveUS8463176B2Improve rendering capabilitiesFeedback loop stabilityFrequency-division multiplex detailsActive radio relay systemsStability indicatingInstability
A method for monitoring feedback loop stability in a wireless repeater includes measuring a gain control metric in the feedback loop of the repeater periodically for a given time period where the gain control metric is indicative of a loop gain of the feedback loop of the repeater; and monitoring the magnitude of the gain control metric to determine the stability of the feedback loop of the repeater. In operation, a large magnitude of the gain control metric indicates instability in the feedback loop of the repeater.
Owner:QUALCOMM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com