A kind of assay method of related substance of methylcobalamin tablet
A technology of related substances and methylcobalamin tablets, applied in the field of analytical chemistry, can solve the problems of difficulty in removing coating, affecting detection accuracy, interference in detection results, etc., achieving good economic benefits and promotion prospects, avoiding detection results deviation, The effect of precise limit control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
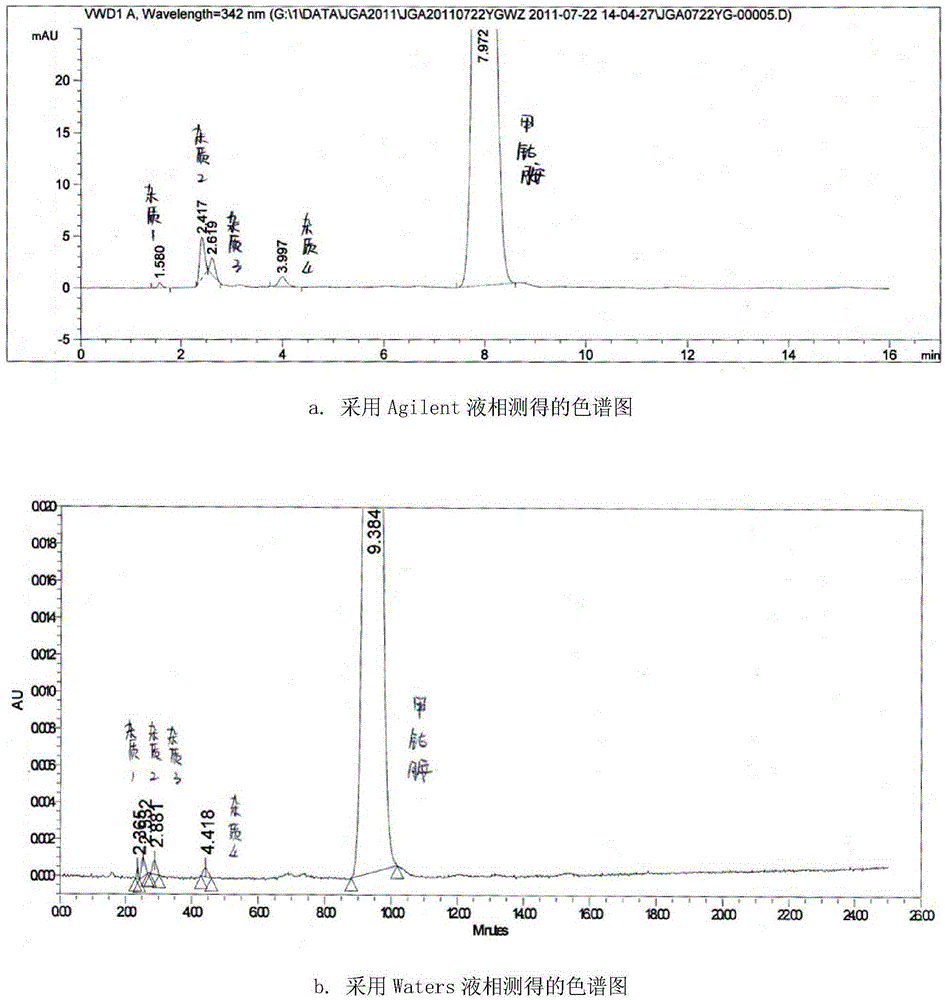
[0042] Directly grind methylcobalamin tablets to fine powder, accurately weigh appropriate amount of fine powder (i.e. equivalent to 2.5 mg of methylcobalamin), put it in a 10ml measuring bottle, add mobile phase acetonitrile-sodium dihydrogen phosphate buffer (volume ratio is 19 :81, containing 0.03mol / L sodium dihydrogen phosphate, with phosphoric acid to adjust the pH value to 3.5), fine powder is dissolved and diluted to scale, shake up, filter, and get the subsequent filtrate as need testing solution;
[0043] Precisely measure an appropriate amount of the subsequent filtrate, and also add mobile phase acetonitrile-sodium dihydrogen phosphate buffer solution (volume ratio is 19:81, containing 0.03mol / L sodium dihydrogen phosphate, and adjust the pH value to 3.5 with phosphoric acid) to dilute to the mark to prepare Become the solution that concentration is 7.5 μ g / ml, as reference substance solution;
[0044] Take another test solution and place it under sunlight for 5-10...
Embodiment 2
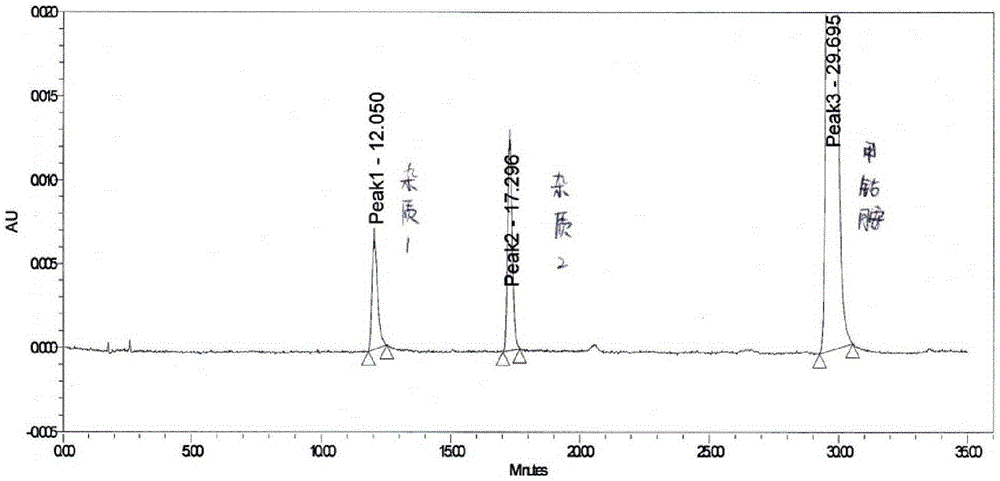
[0051] Directly grind methylcobalamin tablets to fine powder, accurately weigh appropriate amount of fine powder (i.e. equivalent to 2.5 mg of methylcobalamin), put it in a 10ml measuring bottle, add mobile phase acetonitrile-sodium dihydrogen phosphate buffer (volume ratio is 19 :81, containing 0.03mol / L sodium dihydrogen phosphate, with phosphoric acid to adjust the pH value to 3.5), fine powder is dissolved and diluted to scale, shake up, filter, and get the subsequent filtrate as need testing solution;
[0052] Precisely measure an appropriate amount of the subsequent filtrate, and also add mobile phase acetonitrile-sodium dihydrogen phosphate buffer solution (volume ratio is 19:81, containing 0.03mol / L sodium dihydrogen phosphate, and adjust the pH value to 3.5 with phosphoric acid) to dilute to the mark to prepare Become the solution that concentration is 7.5 μ g / ml, as reference substance solution;
[0053] Same as the operation method of Example 1, adopt high performan...
Embodiment 3
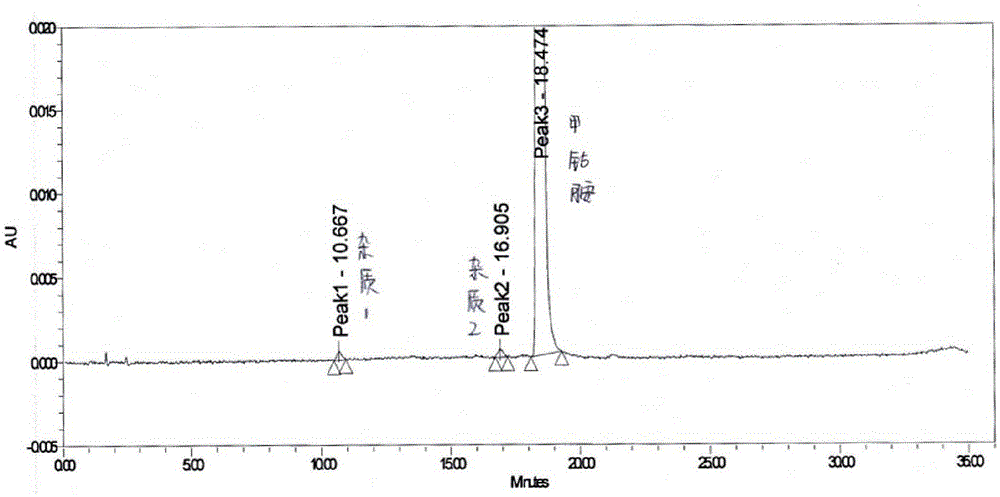
[0055] Directly grind methylcobalamin tablets to fine powder, accurately weigh appropriate amount of fine powder (i.e. equivalent to 2.5 mg of methylcobalamin), put it in a 10ml measuring bottle, add mobile phase acetonitrile-sodium dihydrogen phosphate buffer (volume ratio is 19 :81, containing 0.03mol / L sodium dihydrogen phosphate, with phosphoric acid to adjust the pH value to 3.5), fine powder is dissolved and diluted to scale, shake up, filter, and get the subsequent filtrate as need testing solution;
[0056] Precisely measure an appropriate amount of the subsequent filtrate, and also add mobile phase acetonitrile-sodium dihydrogen phosphate buffer solution (volume ratio is 19:81, containing 0.03mol / L sodium dihydrogen phosphate, and adjust the pH value to 3.5 with phosphoric acid) to dilute to the mark to prepare Become the solution that concentration is 7.5 μ g / ml, as reference substance solution;
[0057] Same as the operation method of Example 1, adopt high performan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com