A method for detecting 4-(1-(2,5-dimethylphenyl)ethyl)-1h-imidazole or/and its hydrochloride
A dimethyl phenyl, detection method technology, applied in the detection field, to achieve the effect of meeting the requirements of impurity control, good durability and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Blank solvent (the following is used as diluent): phosphate buffer-methanol (60:40, v / v), water or acetonitrile-water (3:7, v / v).
[0068] Get the finished product of dexmedetomidine, add corresponding diluent to dissolve and make a solution containing about 0.5mg of dexmedetomidine in every 1ml, as need testing solution; precision measures appropriate amount of need testing solution, dilute with corresponding The solution was diluted to make a solution containing about 1 μg of dexmedetomidine per 1 ml, as a control solution. Accurately weigh appropriate amounts of dexmedetomidine hydrochloride and YM-Z6 reference substance, dissolve with corresponding diluents and make solutions containing about 0.5 mg of dexmedetomidine and about 0.5 μg of YM-Z6 in each 1 ml, as System Suitability Solution.
[0069] Measured according to high-performance liquid chromatography (Chinese Pharmacopoeia 2015 Edition Four General Rules 0512), accurately measure 20 μl of the system suitabil...
Embodiment 2
[0070] Embodiment 2 Screening of detection wavelength
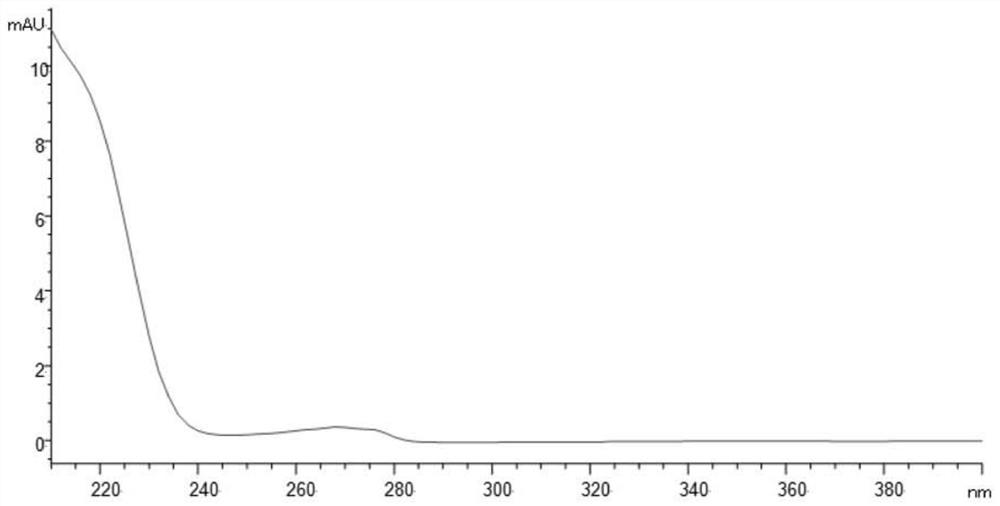
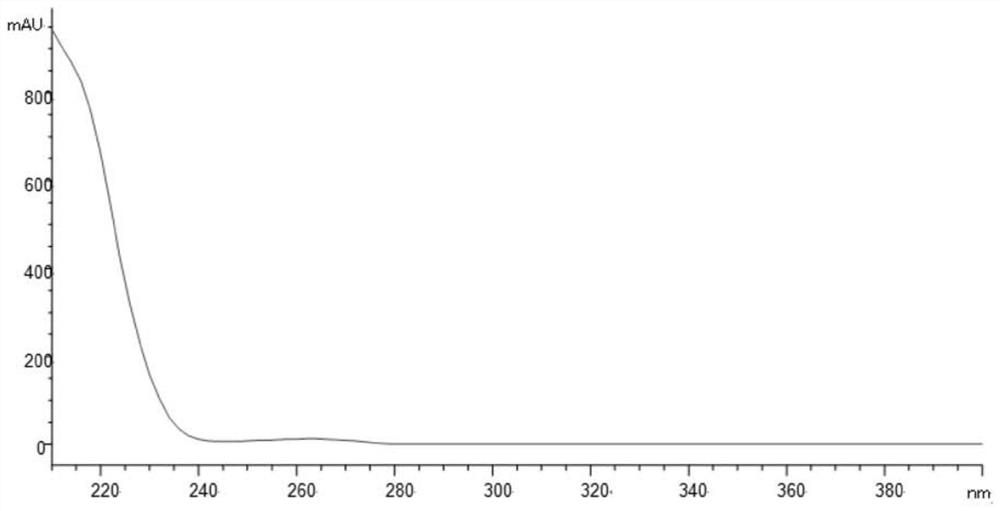
[0071] The ultraviolet absorption spectrum of impurity YM-Z6 is shown in figure 1 , the UV absorption spectrum of the main peak dexmedetomidine is shown in figure 2 .
[0072] It can be seen from the scanning spectrum that the ultraviolet absorption of the main component and impurity YM-Z6 is terminal absorption, and the detection wavelength is set at 220nm with reference to the detection wavelength of the USP related substance method.
Embodiment 3
[0073] The screening of embodiment 3 chromatographic conditions
[0074] (1) Refer to the chromatographic conditions of the related substance method of dexmedetomidine hydrochloride USP standard (USP40s1):
[0075] Mobile phase: phosphate buffer (weigh 0.71 g of disodium hydrogen phosphate and dissolve in 1 L of water, adjust the pH value to 7.0 with 16 g / L sodium dihydrogen phosphate dihydrate)-methanol (40:60);
[0076] Chromatographic column: Agilent InfinityLabPoroshell 120EC-C18, 4.6×150mm, 4μm;
[0077] Flow rate: 1ml / min, detection wavelength: 220nm;
[0078] Column temperature: 25°C, injection volume: 100μl;
[0079] Diluent: Phosphate Buffer-Methanol (60:40).
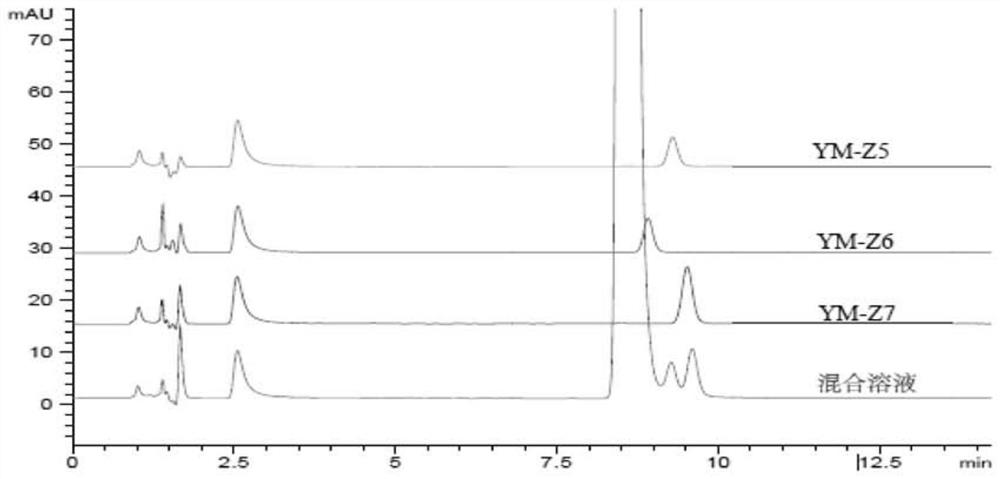
[0080] Depend on image 3 It can be seen that YM-Z6 overlaps with the main peak under this method, indicating that YM-Z6 and dexmedetomidine cannot be separated under this method.
[0081] (2) Referring to the related substance method of dexmedetomidine hydrochloride USP standard (USP40s1), replace differe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com