A kind of photolysis impurity of melphalan and its salt and its hplc detection method
A detection method, the technology of melphalan, is applied in the field of photolysis impurities and HPLC detection, which can solve the problems of inability to monitor PD1 and PD2 at the same time, and inability to accurately quantify, to improve detection sensitivity and durability, and prolong retention time. , Control the effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Discovery and identification of photodegradable impurities
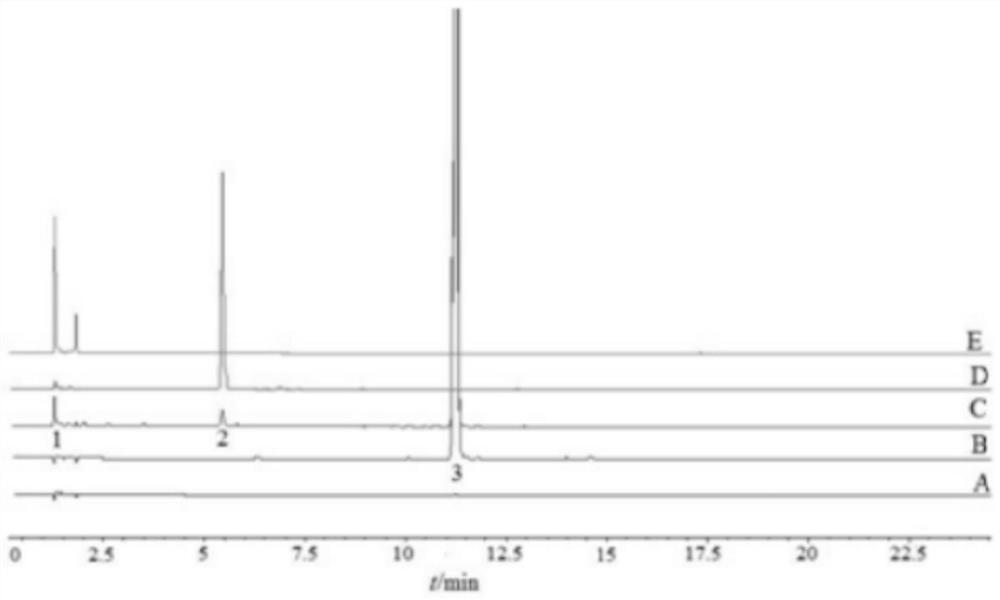
[0040] Chromatographic conditions (refer to Pharmacopoeia method): the chromatographic column is Waters Atlantis T3 column (4.6mm×150mm, 3μm); mobile phase A is 0.01mol / L ammonium acetate solution (adjusted to pH 4.6 with acetic acid), mobile phase B is acetonitrile, gradient The elution is as follows: 0min(95%A-5%B)→20min(40%A-60%B)→25min(40%A-60%B); the flow rate is 1.5ml / min; the injection volume is 10μl; The detection wavelength was 260 nm; the column temperature was 35 °C.
[0041] Place melphalan hydrochloride for 10 days under light conditions (4500lx ± 500lx), dissolve it with methanol and dilute it to a solution of 0.5 mg / ml, and inject and analyze according to the above chromatographic conditions. Chromatograms from melphalan hydrochloride illuminated samples ( figure 1 ), it can be seen that melphalan hydrochloride will produce two large degradation impurities under light conditions, wh...
Embodiment 2
[0044] Example 2: Synthesis of 4-(2-chloroethylamino)-L-phenylalanine hydrochloride
[0045] The synthetic route is as follows:
[0046]
[0047]Dissolve 2 g of N,N-phthaloyl-4-amino-L-phenylalanine ethyl ester (compound A) in 20 ml of ethanol, add 1 ml of triethylamine and 0.8 g of bromoethanol, and react at room temperature for 24 After 1 hour, ethanol was evaporated under reduced pressure, and then a large amount of water was added, extracted with dichloromethane, the organic phase was collected, and 1.7 g of compound B was obtained by column chromatography. Dissolve 1.5 g of compound B in 15 ml of dichloromethane, add 0.8 ml of phosphorus oxychloride, react at 30° C. for 5 hours, and remove the dichloromethane by rotary evaporation to obtain 1.3 g of compound C. Compound C was reacted with concentrated hydrochloric acid under reflux for 12 hours, then stirred at low temperature in ethanolic hydrogen chloride solution for 1 hour, and the solvent was removed by rotary ev...
Embodiment 3
[0049] Example 3: Determination of Photodegradation Impurities by the Method of the Invention
[0050] 1. Chromatographic conditions
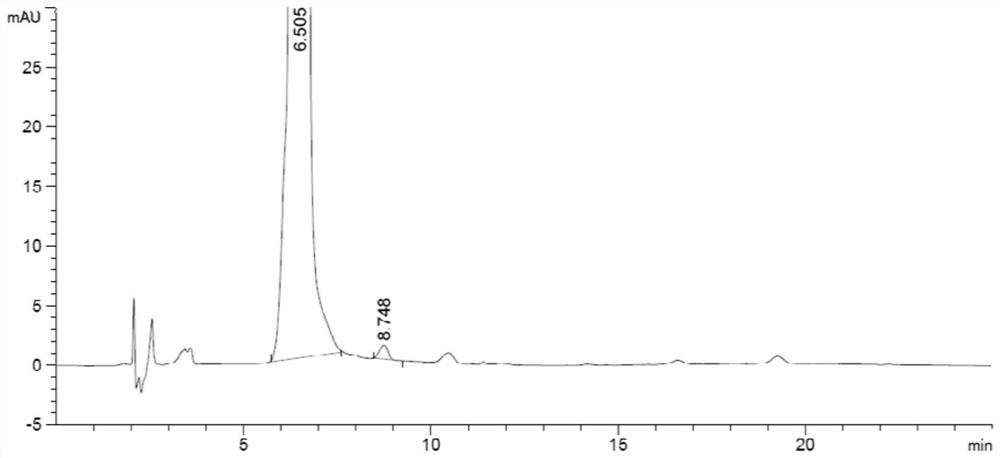
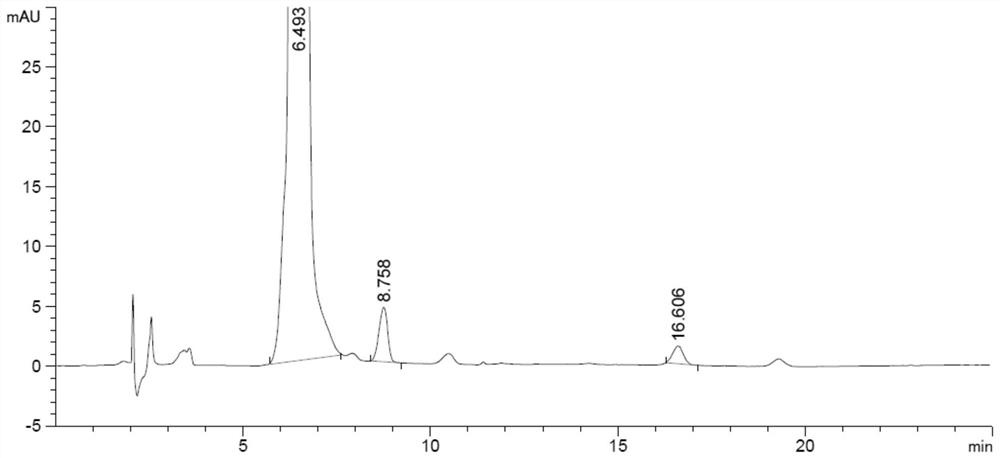
[0051] Chromatographic column: Atlantis HILIC column (4.6mm×150mm, 3μm); mobile phase: 0.1mol / L ammonium formate solution (formic acid adjusted to pH 3.0): acetonitrile=13:87; flow rate: 1.0ml / min; injection volume: 20μl; column temperature: 35°C; detection wavelength: 260nm. Under the chromatographic conditions, the photodegradation impurities PD1, PD2 and other impurities in melphalan hydrochloride can be completely separated.
[0052] 2. Preparation of Solutions
[0053] Impurity stock solution: Precisely weigh appropriate amounts of impurities PD1 and PD2 respectively, dissolve and dilute them with methanol to obtain an impurity stock solution with a concentration of 25 μg / ml.
[0054] Reference solution: Take the impurity stock solution and quantitatively dilute it with methanol to a solution of 1.5 μg / ml.
[0055] Test solution: Accur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com