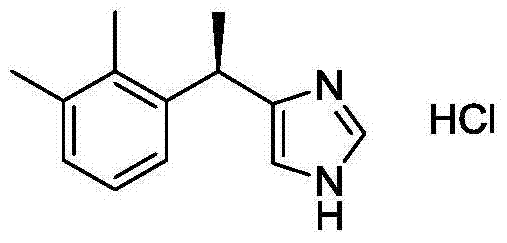
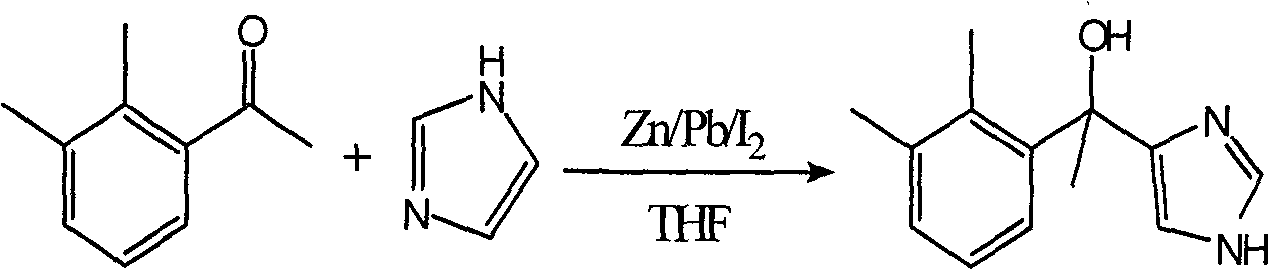Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
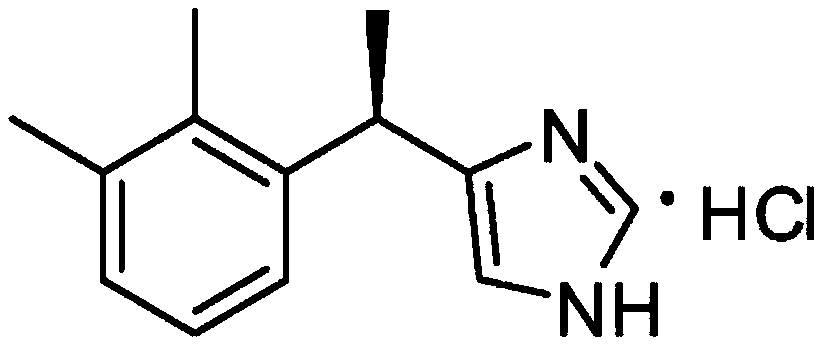
62 results about "Dexmedetomidine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
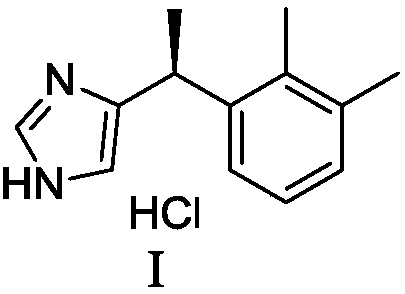
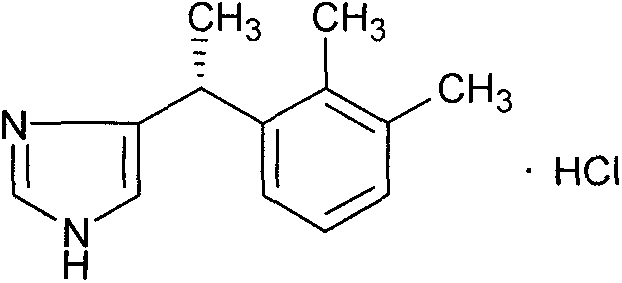
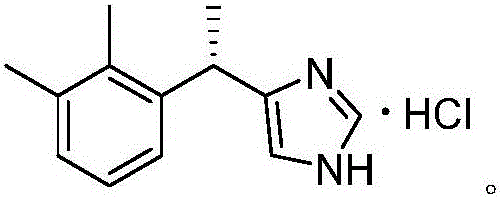
New method for preparing dexmedetomidine hydrochloride
The invention provides a new method for preparing dexmedetomidine hydrochloride. According to the method, Lewis acid is used as a catalyst, and racemized medetomidine is prepared by a Friedel-Crafts reaction; and the racemized medetomidine is resolved by (+)-di-p-toluoyl-tartaric acid [(+)-DDTA] to obtain dexmedetomidine. The dexmedetomidine is salified in hydrochloric acid to obtain dexmedetomidine hydrochloride.
Owner:北京华禧联合科技发展有限公司
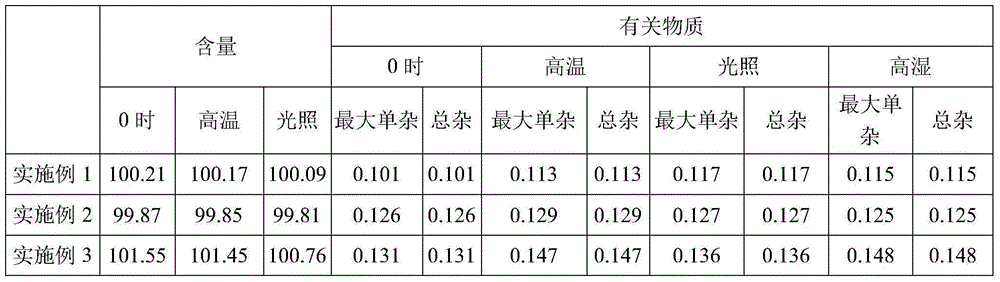
Dexmedetomidine hydrochloride injection and preparation process thereof
ActiveCN105168122ASimple preparation processLow costOrganic active ingredientsNervous disorderActive componentDrugs preparations
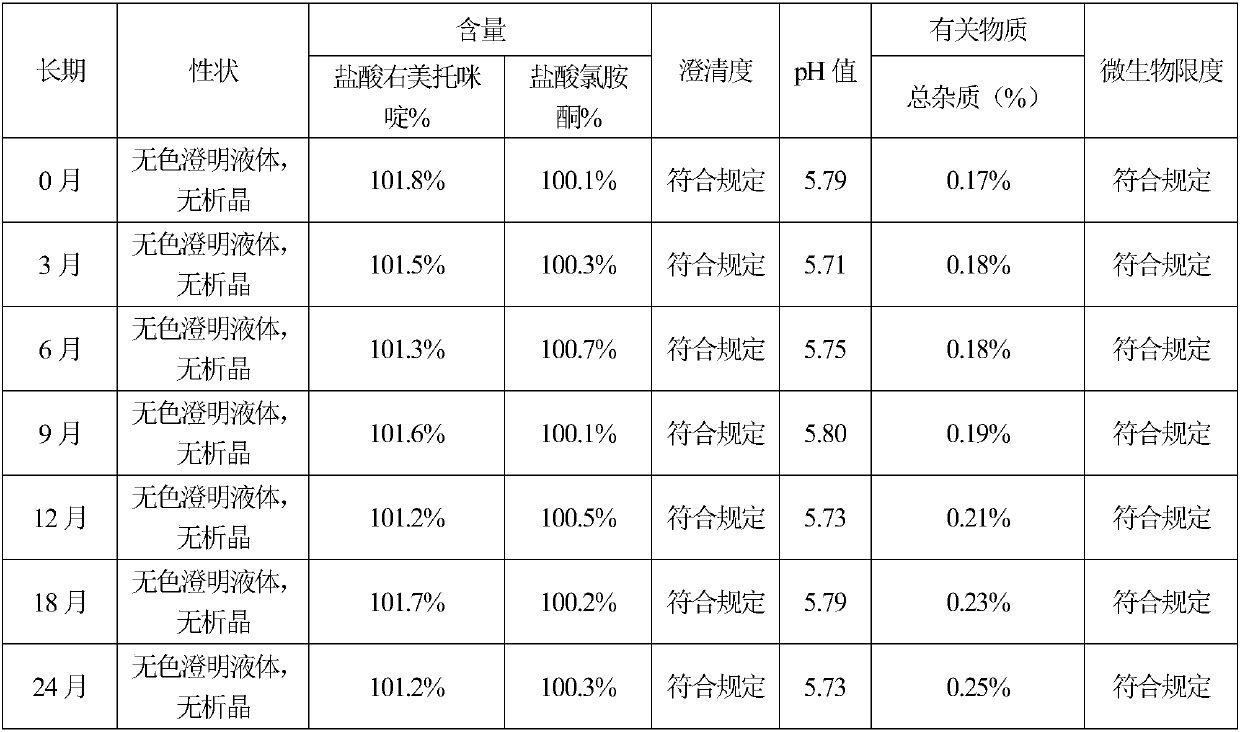
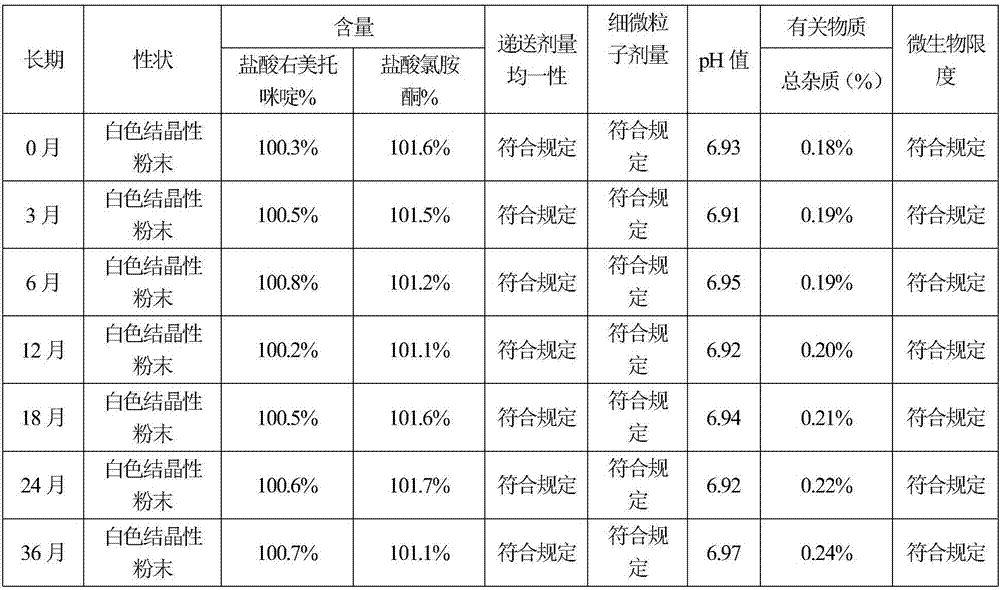
The invention belongs to the technical field of drug preparation, and particularly relates to dexmedetomidine hydrochloride injection and a preparation process thereof. The dexmedetomidine hydrochloride injection comprises main materials and water for injection. The main materials comprise an active component and auxiliary materials. The auxiliary materials comprise an osmotic pressure modifier and a metal ion complexing agent. Dexmedetomidine hydrochloride serves as the active component. Sodium chloride serves as the osmotic pressure modifier. Compared with the prior art, the dexmedetomidine hydrochloride injection has the advantages that pH, content and related substance results of all embodiments are still qualified after the dexmedetomidine hydrochloride injection is subjected to high temperature, illumination and acceleration for 6 months and 24 months, and it is proved that the whole product is stable in quality. In addition, the dexmedetomidine hydrochloride injection is simple in preparation process, low in cost and beneficial to industrial production.
Owner:CISEN PHARMA
Preparation of dexmedetomidine hydrochloride key intermediate
The invention relates to a preparation method of dexmedetomidine hydrochloride key intermediate, an [alpha]2 receptor agonist, namely 1-(2,3-dimethyl)-1-(1H-imidazole-4-yl)ethanol (I). The preparation method is characterized in that reformastsky reaction is adopted, 4-iodoimidazol (II) can be reacted with 2,3-dimethyl acetophenone (III) fast at No.1 position without the need of protection to result in target product; the preparation method has the advantages of easy control of reaction conditions, simple operation, high quality and yield of the product and great availability of raw materials, and ensures the quality of the final synthetic product, namely dexmedetomidine hydrochloride.
Owner:北京华禧联合科技发展有限公司
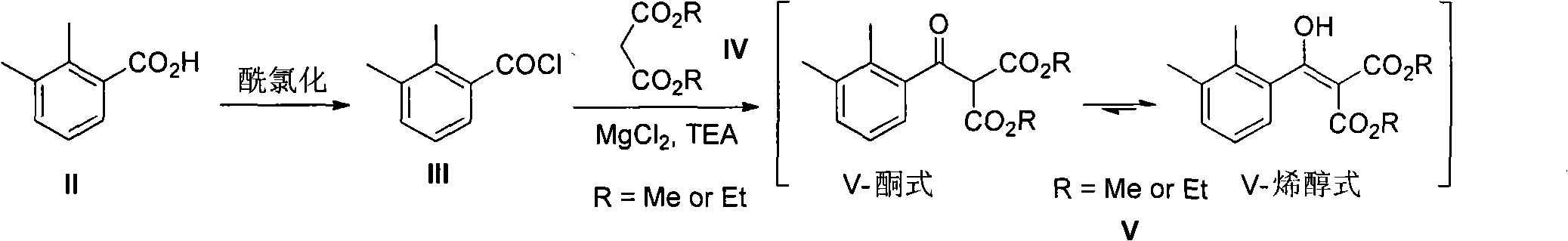
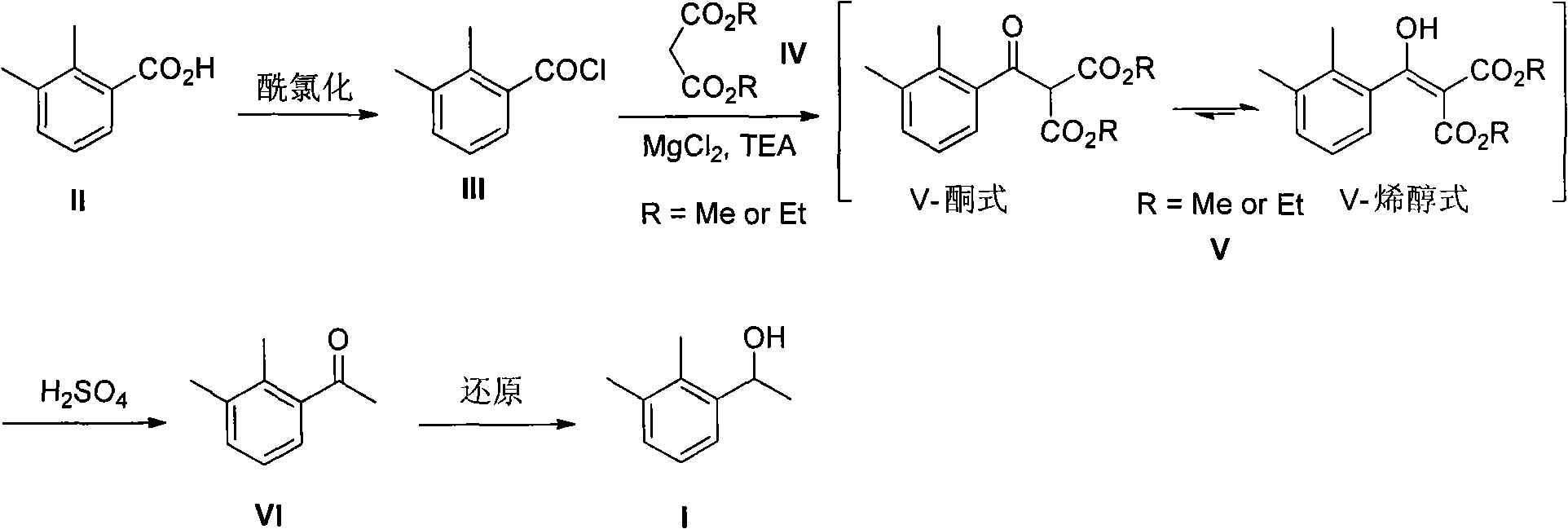
2-(2,3-dimethyl phenyl) diester malonate, preparation method thereof, and application thereof
ActiveCN102249921AReduce usageMild method conditionsOrganic compound preparationCarboxylic acid esters preparationMalonateReducing agent
The invention discloses a type of 2-(2,3-dimethyl phenyl) diester malonate, a preparation method thereof, and an application thereof in an intermediate 1-(2,3-dimethyl phenyl) ethanol used for synthesizing dexmedetomidine hydrochloride. According to the invention, 2,3-dimethylbenzoic acid is adopted as an initial raw material. The initial raw material is chlorized into 2,3-dimethylbenzoyl chloride. 2,3-dimethylbenzoyl chloride is subject to a reaction with diester malonate under the existences of triethanolamine and magnesium chloride anhydrous, such that 2-(2,3-dimethyl phenyl) diester malonate is obtained. 2-(2,3-dimethyl phenyl) diester malonate is hydrolyzed with sulfuric acid, such that 1-(2,3-dimethyl phenyl)ethanone is obtained. 1-(2,3-dimethyl phenyl)ethanone is then reduced by a reducing agent, such that 1-(2,3-dimethyl phenyl)ethanol is obtained. The operation of the method is simple and convenient; the raw materials of the method are cheap and are easy to obtain; the reaction conditions are mild; and the purity of the finished products is high. With the method provided by the invention, special separating and purifying are not required. The method is suitable to be applied in large-scale productions.
Owner:SHANGHAI SYNFARM PHARMA TECH
Preparation method of pre-filled dexmedetomidine hydrochloride injection
InactiveCN103284945ASolve pollutionSolve usabilityOrganic active ingredientsNervous disorderMedicineNitrogen
The invention discloses a preparation method of a pre-filled dexmedetomidine hydrochloride injection. The preparation method of the pre-filled dexmedetomidine hydrochloride injection comprises the following steps of: A, preparing 80% of prescribed dose of water for injection, adding the prescribed dose of dexmedetomidine hydrochloride, stirring to dissolve for 5-8 minutes, then adding the water for injection to full dose, carrying out convection, and stirring and dissolving for 8-10 minutes; B, filtering a solution obtained by step A by virtue of a filter, and detecting an intermediate; and C, carrying out nitrogen filling, prefilling, encapsulation and sterilization on a solution obtained by step B, thus the pre-filled dexmedetomidine hydrochloride injection is obtained. The preparation method of the pre-filled hydrochloric acid dexmedetomidine injection has the advantages that concept is skilful, operation is simple, the problem that the dexmedetomidine hydrochloride injection is polluted in clinical application and inconvenient to use can be effectively solved, and rapidity and safety of an operation process can be guaranteed.
Owner:SICHUAN BAILI PHARM CO LTD
Preparation method of dexmedetomidine hydrochloride and its intermediate
ActiveCN108147999AShort route stepsHigh molar yieldSilicon organic compoundsPreparation by halogen halide additionBenzeneHydrogen
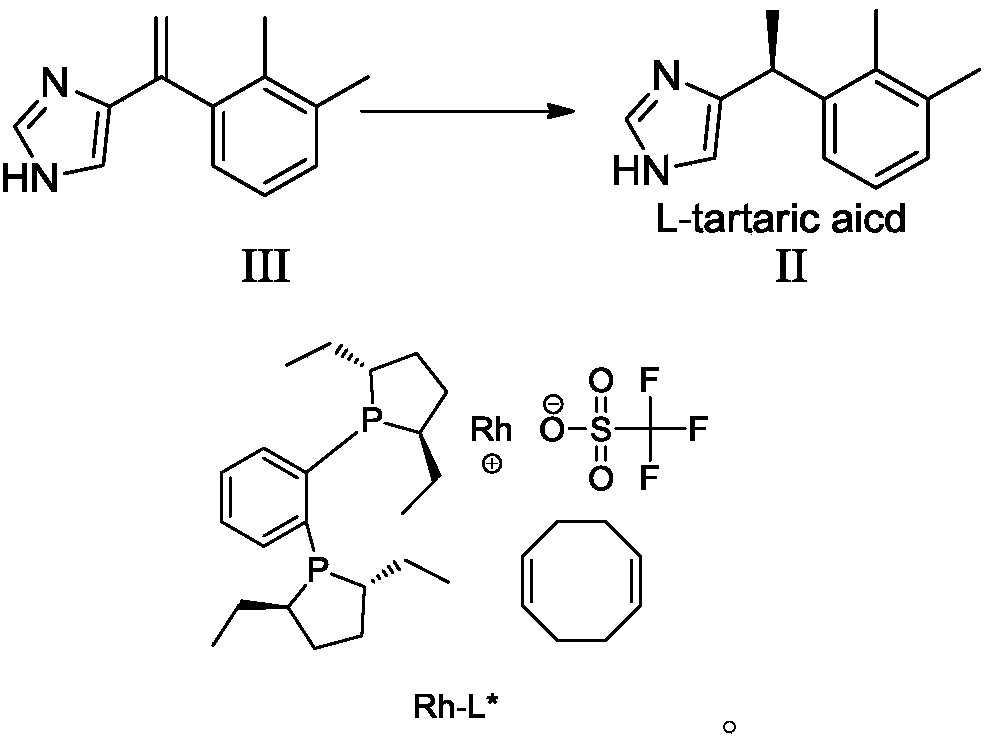
The invention discloses a preparation method of dexmedetomidine hydrochloride and its intermediate. A preparation method of dexmedetomidine L-tartrate comprises the steps of subjecting dexmedetomidineintermediate III and hydrogen to reduction reaction in an organic solvent in the presence of a chiral catalyst, and subjecting the reduced product and tartaric acid to neutralization reaction to obtain dexmedetomidine L-tartrate II, wherein the chiral catalyst is (+)-1,2-bis(2S-5S)-diethylphospholano-benzene(1,5-cyclooctadiene)rhodium trifluoromethanesulfonate. The preparation method herein has ashort step path, has no need for chiral splitting, and has high total molar yield; the product prepared herein has high purity, reaches the standard for bulk pharmaceutical chemicals and is suitablefor industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Special purpose fluid dispenser
InactiveUS7896843B2Easy and inexpensive to manufactureAutomatic syringesMedical devicesWrong drugDelivery system
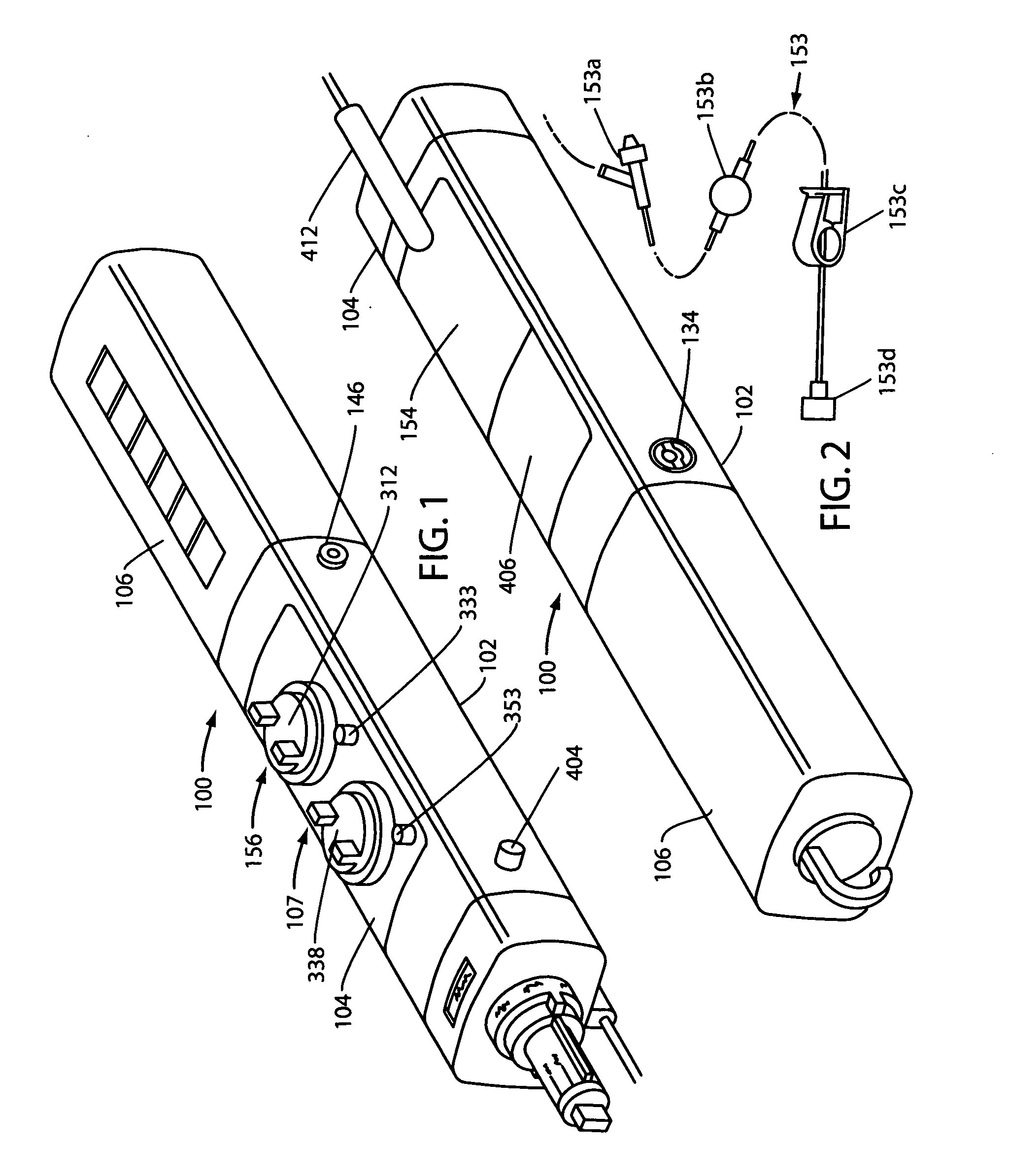
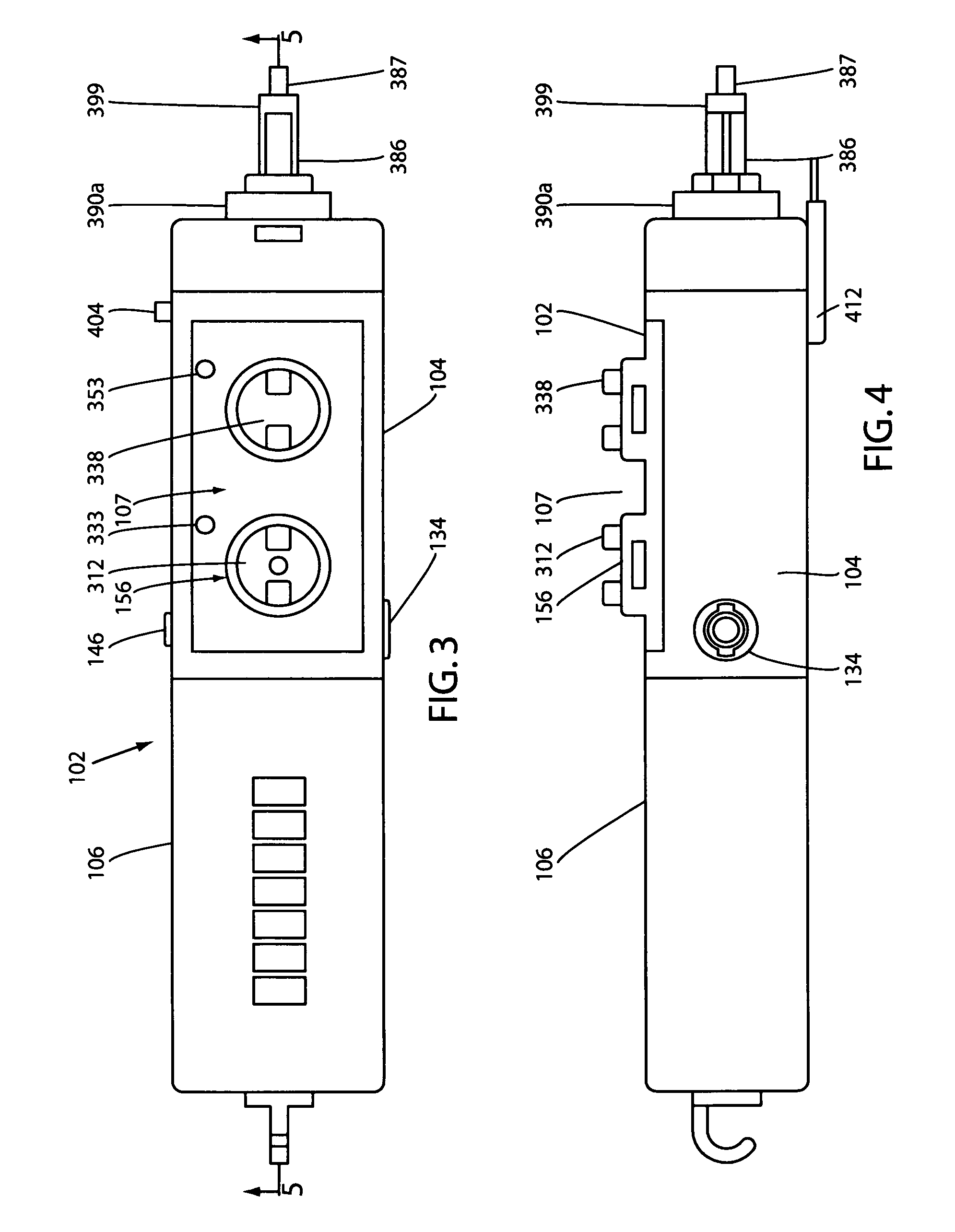
A compact, nonelectric fluid dispenser for use in controllably dispensing beneficial agents such as propofol and dexmedetomidine hydrochloride to patients. The dispenser includes a fluid flow control assembly that precisely controls the flow of the medicament solution to the patient and embodies a collapsible drug container that can be filled in the field with the beneficial agents to be delivered to the patient. The unit-dose fluid dispenser of the invention is presented in a sterile and aseptic manner, where the drug has been pre-filled in the system, so that the practitioner cannot mistakenly give the wrong drug to the patient. The dispenser uniquely provides a more efficient medicament delivery system for procedure rooms, such as the endoscopy center, so that a greater number of patients can be treated per day at a higher standard of care with increased profits for the healthcare provider.
Owner:BIOQ PHARMA
Nasal drops used for anesthesia and preparation method of nasal drops
InactiveCN107693485ANo painImprove complianceOrganic active ingredientsInorganic non-active ingredientsAntioxidantPh regulation
The invention provides nasal drops used for anesthesia. The nasal drops are characterized in that dexmedetomidine hydrochloride injection and ketamine hydrochloride are adopted as the raw materials, an osmotic pressure regulator, a bacteriostatic agent, an antioxidant, a cosolvent and a pH value regulator with certain amount are added, the steps including concentrated preparation, diluted preparation, filling, sterilizing and externally packaging are carried out, and thus the nasal drops are obtained. For the nasal drops used for anesthesia, a special administration channel does not need to beestablished, so that no pain exists during the medication process of a patient, the compliance is good, the stability is good during the storage process of the product, deterioration due to crystallization does not exists, within the period of validity, the pH of the solution basically has no change, the microbial limit is qualified, the validity period of the product is as long as 24 months or alonger time, the increased amount of impurities during the preparation process is small, the increased amount of impurities during the whole preparation process is only 0.01%, the preparation processis simple and feasible, and the nasal drops are worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
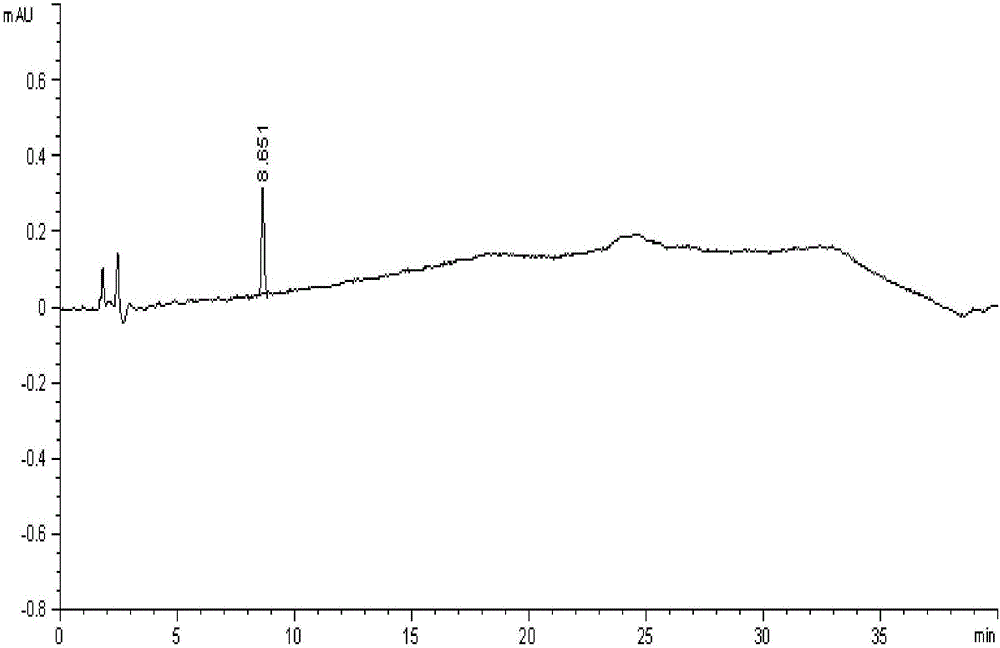
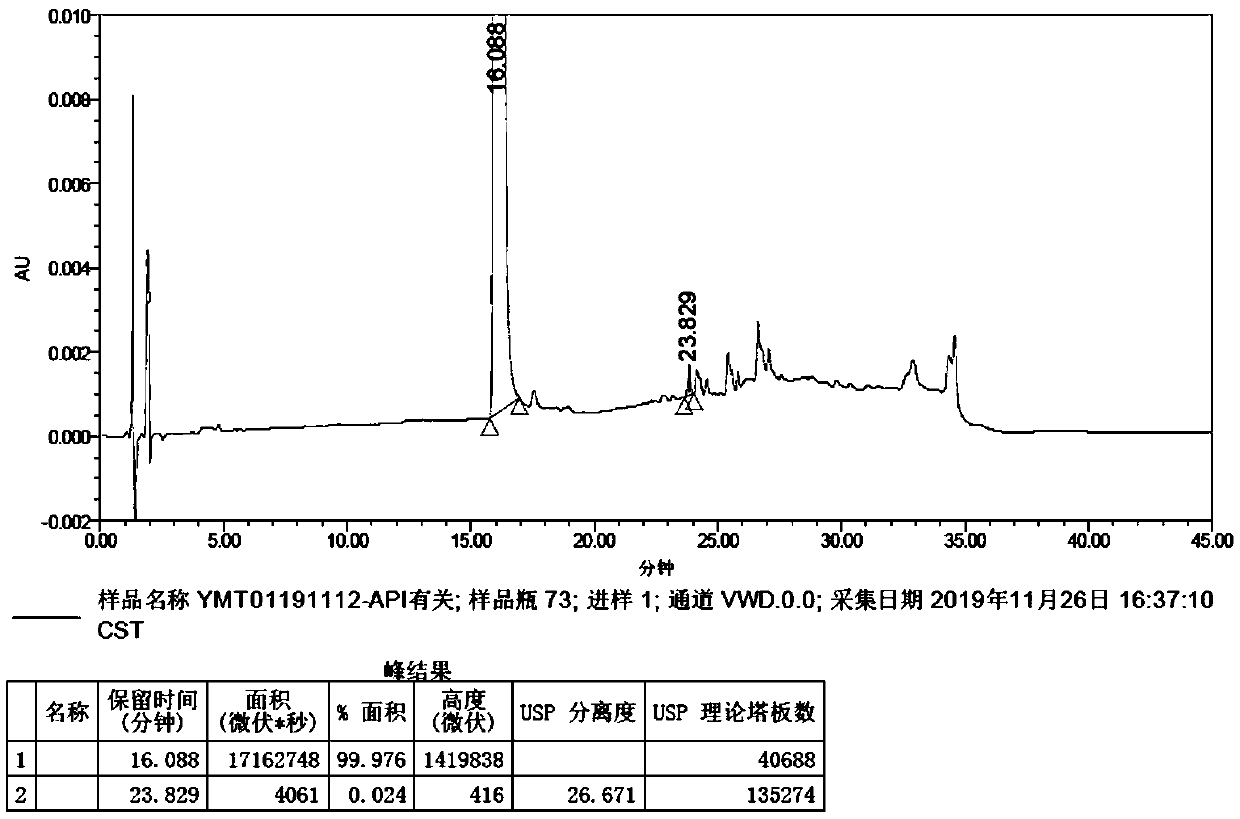
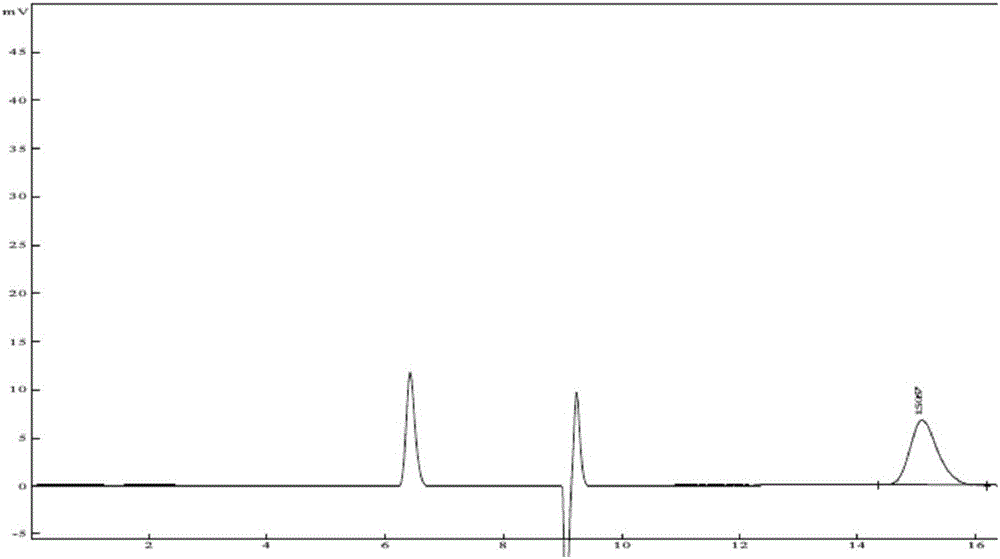
Method of measuring content of related substance in dexmedetomidine hydrochloride active ingredient
InactiveCN107402262AAccurately measure the content of substancesImprove quality controlComponent separationBULK ACTIVE INGREDIENTDrug product
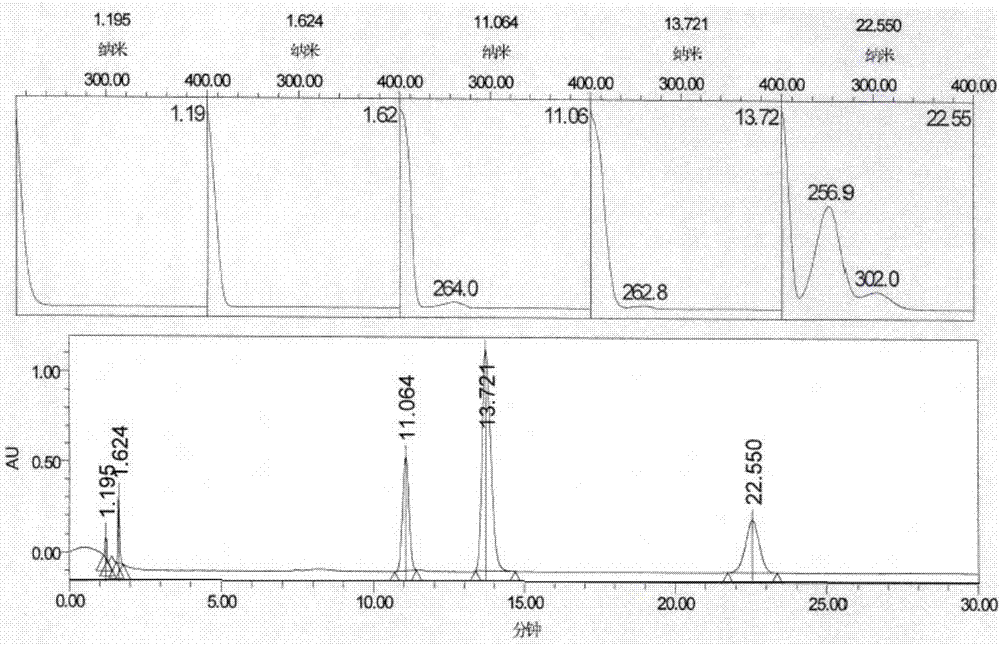
The invention provides a method of measuring the content of related substance in dexmedetomidine hydrochloride active ingredient. The method comprises the following steps: analyzing the dexmedetomidine hydrochloride active ingredient by means of liquid chromatography to obtain a chromatogram conveniently; and determining the content of related substance in the dexmedetomidine hydrochloride active ingredient based on the chromatogram. The method can effectively measure impurities, initial materials and intermediates probably introduced in a synthetic process of dexmedetomidine hydrochloride and effectively control the quality of a dexmedetomidine hydrochloride drug. The method has the characteristics of effectiveness, sensitivity, specificity and accuracy.
Owner:YICHANG HUMANWELL PHARMA
Special purpose fluid dispenser with pre-filled reservoir
ActiveUS8100890B2Easy and inexpensive to manufactureAutomatic syringesPharmaceutical delivery mechanismWrong drugBiomedical engineering
A compact, nonelectric fluid dispenser for use in controllably dispensing beneficial agents such as propofol and dexmedetomidine hydrochloride to patients. The dispenser includes a fluid flow control assembly that precisely controls the flow of the medicament solution to the patient and embodies a collapsible, pre-filled drug container that contains the beneficial agents to be delivered to the patient. The unit-dose fluid dispenser of the invention is presented in a sterile and aseptic manner, where the drug has been pre-filled in the system, so that the practitioner cannot mistakenly give the wrong drug to the patient. The dispenser uniquely provides a more efficient medicament delivery system for procedure rooms, such as the endoscopy center, so that a greater number of patients can be treated per day at a higher standard of care with increased profits for the healthcare provider.
Owner:BIOQ PHARMA
Method for preparing high-purity dexmedetomidine hydrochloride crystal from high-purity intermediate crystal
InactiveCN105175340AEasy to storeOptically-active compound separationOrganic racemisationElemental analysisImpurity
The invention discloses a high-purity intermediate crystal of dexmedetomidine, a preparation method of the high-purity intermediate crystal, a high-purity dexmedetomidine hydrochloride crystal prepared from the high-purity intermediate crystal and a method for preparing the high-purity dexmedetomidine hydrochloride crystal from the high-purity intermediate crystal. The prepared intermediate crystal is dextro-4[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole-L-(-) malate which has the purity of 99.9%, the total related substance impurity content of less than 0.10%, the single impurity content of less than 0.05%, the optical purity of 100.0% and the elemental analysis and theoretical value difference of less than 0.3% and is stable in property and easy to store; and the high-purity dexmedetomidine hydrochloride crystal, namely, 4-[1-2,3-dimethylphenyl)ethyl]-1H-imidazole hydrochloride, prepared from the intermediate crystal prepared by using the method, has the purity of 99.9%, the total related substance impurity content of less than 0.10%, the single impurity content of less than 0.05%, the optical purity of 100.0% and the elemental analysis and theoretical value difference of less than 0.3% and is stable in property and easy to store.
Owner:HAINAN GENERAL & KANGLI PHARMA
Anesthetic nasal spray and preparation method thereof
InactiveCN109620802AEasy to acceptLow costOrganic active ingredientsAerosol deliveryPoor complianceSufentanil Citrate
The invention discloses an anesthetic nasal spray and a preparation method thereof. The anesthetic nasal spray can be used for overcoming local anesthesia injection pain in a minor operation of an adult outpatient clinic, can fully solve the problems of preoperative and intraoperative tension, poor compliance and poor sensitivity; the anesthetic nasal spray is prepared from, by weight, 3-5 ml of adexmedetomidine hydrochloride injection, 0.5-2 ml of a sufentanil citrate injection and 8-10 ml of normal saline. The preparation method of the anesthetic nasal spray comprises the following steps that a, the dexmedetomidine hydrochloride injection is sucked by a medical syringe A; b, the sufentanil citrate injection is sucked by a medical syringe B; c, the normal saline is sucked by the medicalsyringe B to dilute the sufentanil citrate injection; d, the liquids in the medical syringe A and medical syringe B are mixed, and then standing is performed for 3-10 minutes.
Owner:杜皓
Method for detecting related substances of dexmedetomidine hydrochloride raw material or preparation
The invention belongs to the technical field of medicine detection, and in particular relates to a method for detecting related substances of a dexmedetomidine hydrochloride raw material or a preparation. The method specifically comprises the following steps: detecting by using a high performance liquid chromatography method, and performing gradient mobile phase elution, wherein the detection wavelength is 220nm; a 50-95% acetonitrile phosphate buffer solution is adopted as a sample solvent; octyl bonded silica gel, octadecyl bonded silica gel or N-oxidized lycopodine M bonded silica gel is adopted as a chromatographic column of packing; a phosphate buffer solution and acetonitrile are adopted as mobile phases; and the phosphate buffer solution is prepared by dissolving 2.64g of diammonium hydrogen phosphate with water, diluting to be 1000ml, and adjusting the pH value to be 7.3 by using phosphoric acid. Compared with the prior art, the detection method provided by the invention is capable of well separating dexmedetomidine hydrochloride from other inert matters, is capable of accurately testing the contents of other impurities, and has the characteristics of being accurate and reliable in result and good in specificity.
Owner:CISEN PHARMA
Invisible nasal sticker preparation for pediatric anesthesia preoperative sedation and preparation method for invisible nasal strip preparation
InactiveCN106727443AImprove permeabilitySpeed up entryOrganic active ingredientsNervous disorderNasal cavitySedation
The invention discloses an invisible nasal sticker preparation for pediatric anesthesia preoperative sedation and a preparation method for the invisible nasal sticker preparation. Such a nasal sticker comprises an elastic biocolloid prepared from a biological skeleton material and a medicinal beta-cyclodextrin inclusion complex and a transparent back sticker. According to the nasal sticker, a high polymer biological material is adopted as a carrier, so that the nasal sticker has the advantages of stable and remarkable efficacy, small and invisible structure, convenience and comfort for use, capability of targeted delivery at an acupoint, fast action and the like. The nasal sticker has the remarkable effects that the nasal sticker preparation is applied to the Yingxiang acupoint or the nasal cavity of a child patient to be operated to make a sedative dexmedetomidine hydrochloride in the nasal sticker preparation rapidly absorbed by the nasal mucosa, so that the aim of sedation before anesthesia without influence on breathing is fulfilled, and the adverse psychological effects of muscular injection or intravenous injection on the child patient are avoided.
Owner:WUHAN UNIV
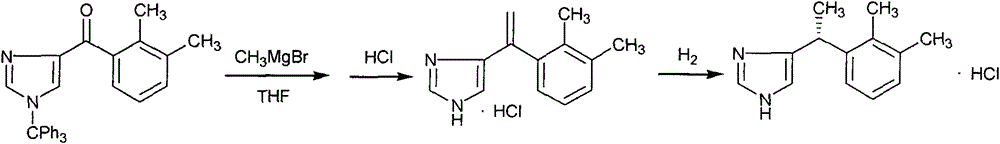
New method for preparing dexmedetomidine hydrochloride key intermediate
InactiveCN104447562AShort stepsEasy to operateOrganic chemistryBulk chemical productionGrignard reagentKetone
The invention provides a method for preparing a dexmedetomidine hydrochloride key intermediate 4-(2,3-dimethyl benzoyl)-1-triphenylmethyl imidazole. The method is characterized in that 1-triphenylmethyl-4-(N-methyl-N-methoxy) imidazole formamide is used as a raw material and reacted with a Grignard reagent of 2,3-dimethyl halogenobenzene to obtain the 4-(2,3-dimethyl benzoyl)-1-triphenylmethyl imidazole. The steps of the method are simple, the reaction can well remain on 'ketone', the yield is high, and the raw materials are rich and convenient, so the method can be effectively used for industrial large-scale production.
Owner:NINGBO TEAM PHARMA
Treatment method for improving stability of dexmedetomidine hydrochloride injection and dexmedetomidine hydrochloride injection
ActiveCN108113986AImprove stabilityImprove water resistanceOrganic active ingredientsNervous disorderMedicineNitrogen
The invention discloses a treatment method for improving the stability of a dexmedetomidine hydrochloride injection and the dexmedetomidine hydrochloride injection. The treatment method comprises thefollowing steps: in the presence of nitrogen, filtering a prepared dexmedetomidine hydrochloride injection by using a micro-pore filter element, cooling to 35-40 DEG C, putting into a glass bottle ofwhich the inner surface is modified by active groups, and performing sterilization, so as to obtain the dexmedetomidine hydrochloride injection, wherein the active groups comprise one or more of amino, sulfydryl, carboxyl and anhydride. By adopting the method, the stability of the dexmedetomidine hydrochloride injection can be improved without any auxiliary material, and the injection is preventedfrom adverse influence caused by the auxiliary material.
Owner:石药银湖制药有限公司
Dry powder inhalant for anesthesia and preparing method thereof
InactiveCN107412204ANo painImprove complianceOrganic active ingredientsPowder deliveryImpurityLubricant
Disclosed is a dry powder inhalant for anesthesia. The dry powder inhalant for the anesthesia is prepared by the following steps that dexmedetomidine hydrochloride injection and ketamine hydrochloride are used as raw materials, a certain amount of carriers, active protective agents, lubricating agents and pH adjusting agents are added, and pretreatment, mixing, drying, total mixing and injected packing are conducted. According to the dry powder inhalant for the anesthesia, special medicine feeding channels do not need to be established, in the medicine taking process, patients are painless, the compliance is good, the stability in the product storing process is good, pH changing and impurity increment in the storing process are low, the impurity increment within the expiry date is only 0.06%, the expiry date of products reaches above 36 months, the flowability of the powder is good, the angle of repose is less than 42 degrees, the content uniformity is good, the content RSD of a plurality of points is less than 1%, the preparing process is simple and easy to operate, and the dry powder inhalant for the anesthesia is worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
Dexmedetomidine hydrochloride injection composition
ActiveCN107412152AFor long-term storageSafe for clinical useOrganic active ingredientsNervous disorderForeign matterSide effect
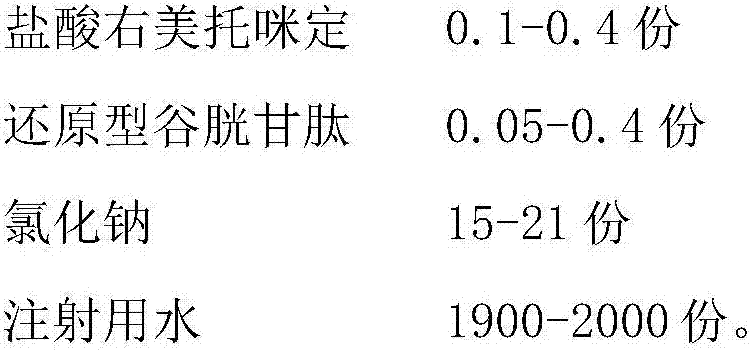
The invention belongs to the technical field of medicines and particularly relates to a dexmedetomidine hydrochloride injection composition which includes: dexmedetomidine hydrochloride, reduced glutathione, sodium chloride and water for injection, wherein the mass ratio of the dexmedetomidine hydrochloride to the reduced glutathione in the injection composition is 0.6:1-4:1. Compared with the prior art, the dexmedetomidine hydrochloride injection composition has good photo-stability, is not liable to generate visible foreign matters, is easy to store for long time and is safe to use clinically, has good treatment effect and is low in side effect. In addition, a preparation process of the dexmedetomidine hydrochloride injection composition is simple and low-cost and has convenience in industrial production.
Owner:广东泽盛药业有限公司
Methods for treating agitation using dexmedetomidine hydrochloride
PendingUS20210267944A1Reduce agitationReduce signOrganic active ingredientsNervous disorderIntravenous routePharmaceutical drug
The present disclosure relates to the treatment of agitation or signs of agitation in certain human subjects, including subjects with a neurodegenerative, neuropsychiatric or opioid withdrawal disorder, by administering dexmedetomidine hydrochloride by the intravenous route.
Owner:BIOXCEL THERAPEUTICS INC
Industrial preparation method of dexmedetomidine hydrochloride
The invention discloses an industrial preparation method of dexmedetomidine hydrochloride, and belongs to the field of medicines. The method comprises the following steps: directly carrying out a Friedel-Crafts alkylation reaction on initial raw materials comprising 1-(2,3-dimethylphenyl)ethanol not subjected to a chlorination (thionyl chloride) reaction and protected imidazole under the catalysis of a Lewis acid to obtain racemic dexmedetomidine, carrying out pre-resolution purification on the racemic dexmedetomidine through a chiral acid, carrying out chiral acid resolution and alkali dissociation, and adding a hydrochloric acid organic solvent to form a salt in order to obtain the dexmedetomidine hydrochloride. The method avoids use of the toxic and corrosive regent thionyl chloride, allows the above product with high chiral and chemical purity to be obtained and the yield to be high, and is suitable for industrial production.
Owner:江苏开元医药有限公司
Special purpose fluid dispenser
InactiveUS20100094203A1Easy and inexpensive to manufactureAutomatic syringesPharmaceutical delivery mechanismWrong drugMedicine
A compact, nonelectric fluid dispenser for use in controllably dispensing beneficial agents such as propofol and dexmedetomidine hydrochloride to patients. The dispenser includes a fluid flow control assembly that precisely controls the flow of the medicament solution to the patient and embodies a collapsible drug container that can be filled in the field with the beneficial agents to be delivered to the patient. The unit-dose fluid dispenser of the invention is presented in a sterile and aseptic manner, where the drug has been pre-filled in the system, so that the practitioner cannot mistakenly give the wrong drug to the patient. The dispenser uniquely provides a more efficient medicament delivery system for procedure rooms, such as the endoscopy center, so that a greater number of patients can be treated per day at a higher standard of care with increased profits for the healthcare provider.
Owner:BIOQ PHARMA
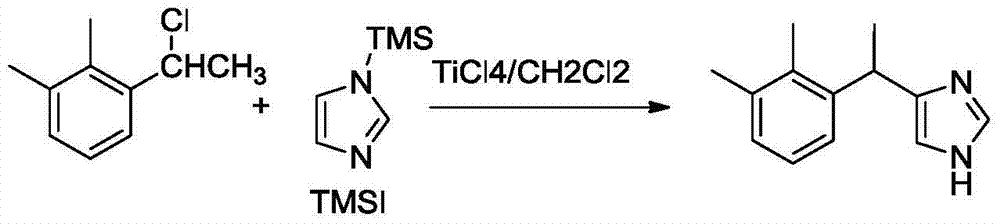
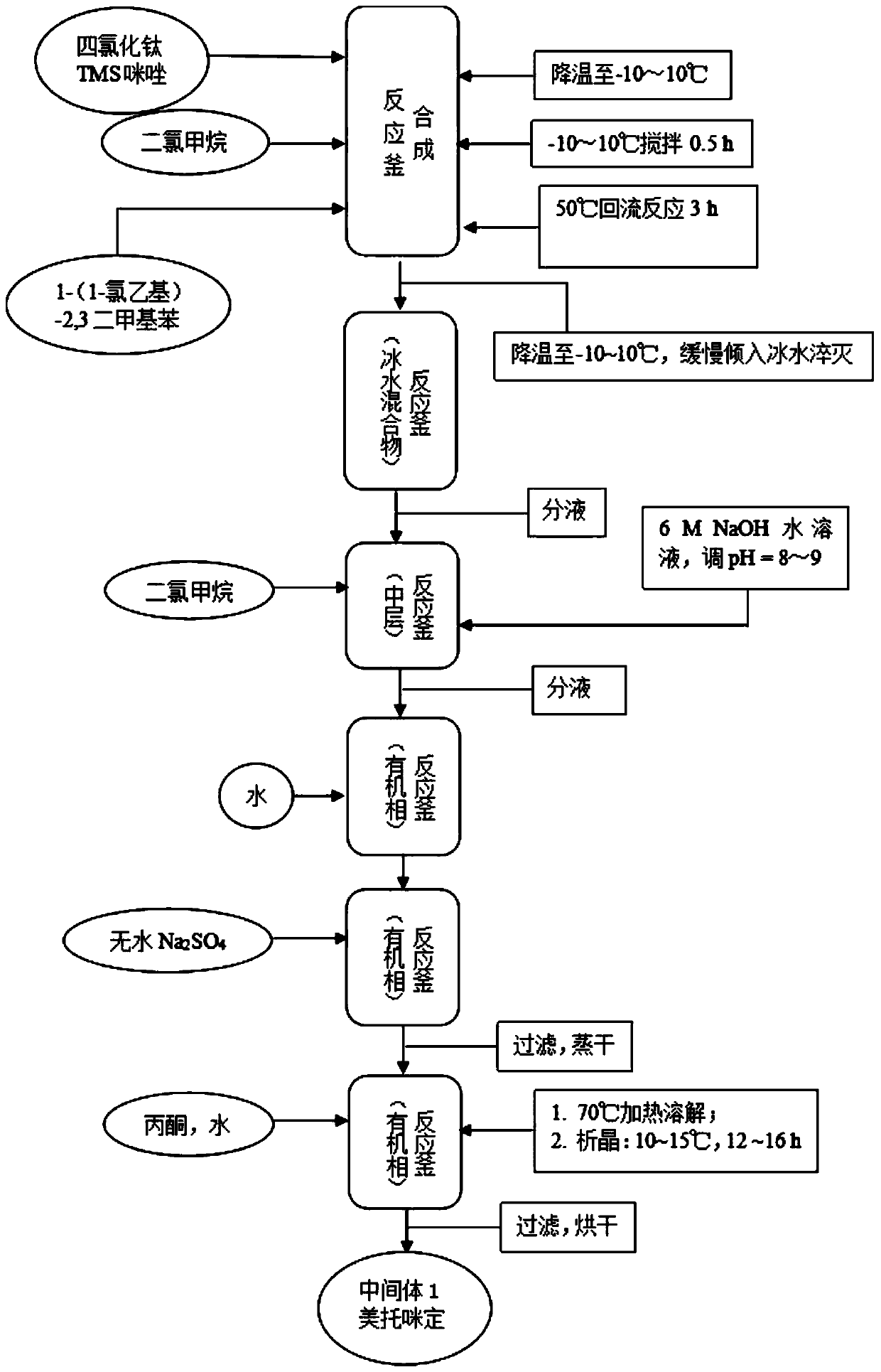
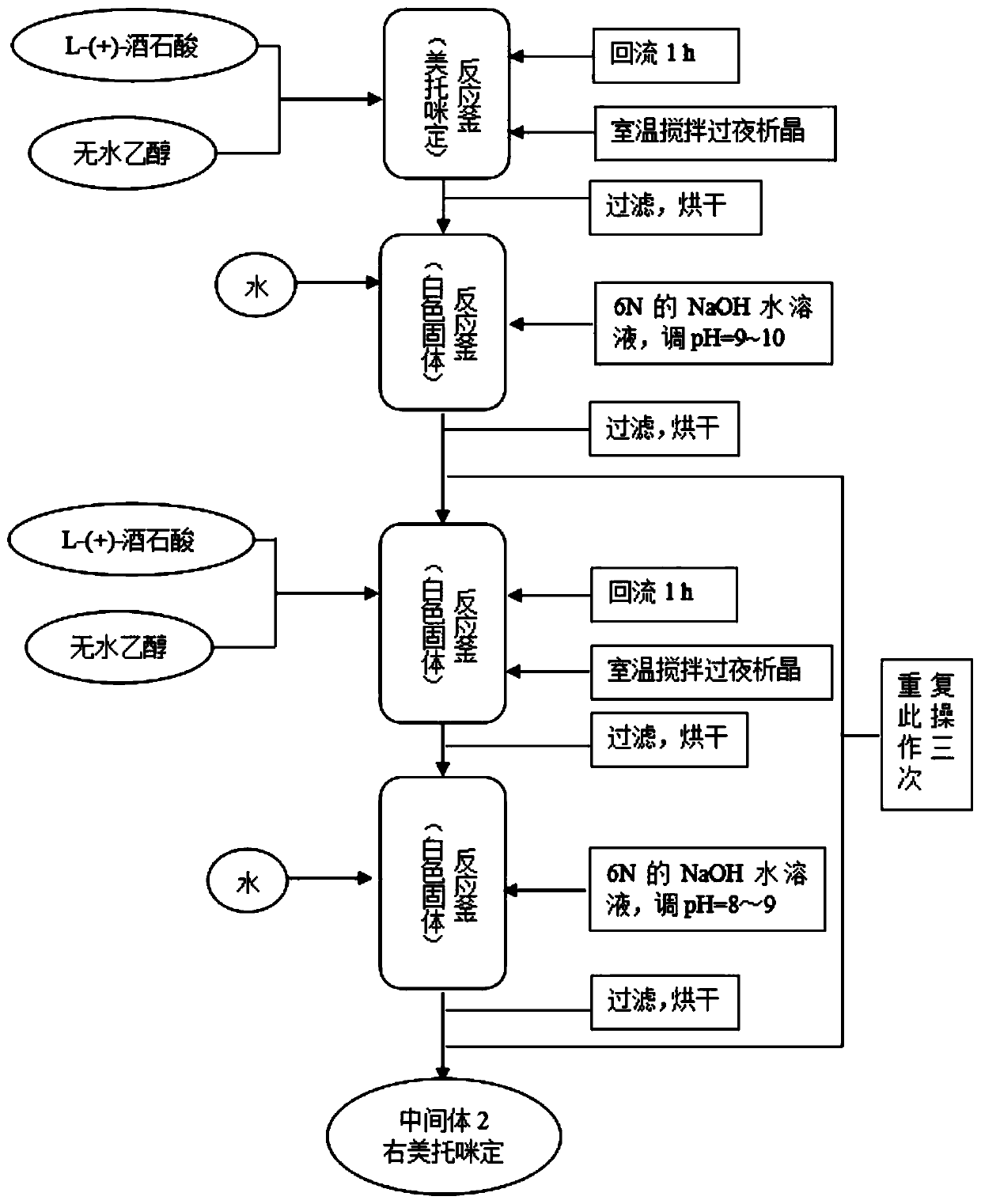
Synthetic process of dexmedetomidine hydrochloride
PendingCN111548308AEasy to operateQuality is easy to controlOrganic chemistry methodsXylyleneDrugs synthesis
The present invention provides a dexmedetomidine hydrochloride synthesis process, and relates to the technical field of drug synthesis, the dexmedetomidine hydrochloride synthesis process uses 1-(1-chloroethyl)-2,3-xylene and trimethylsilylimidazole as starting materials, and alkylation, resolution, dissociation and salification reactions are performed to synthesize the dexmedetomidine hydrochloride. According to the method, L-tartaric acid is used as a resolving agent, and the resolving efficiency is improved by controlling the proportion of the resolving agent and the number of resolving times; a one-step method is adopted for free salification, intermediate quality control and purification steps are not needed, the purity of the prepared finished product is larger than 99.8%, the isomeris not larger than 0.2%, through process optimization, the efficiency of the synthesis process is remarkably improved, and the obtained finished product directly meets the commercial production requirements of raw material medicines.
Owner:TIANJIN PHARMA GROUP XINZHENG
Dexmedetomidine hydrochloride lyophilized powder injection and preparation method
InactiveCN108743551AExcellent indicatorsEasy to storePowder deliveryOrganic active ingredientsFreeze-dryingMiddle molecular weight
The invention relates to a dexmedetomidine hydrochloride lyophilized powder injection and a preparation method, and belongs to the field of pharmaceutical preparations. The dexmedetomidine hydrochloride lyophilized powder injection comprises, by weight, 1 part of dexmedetomidine hydrochloride and 2-20 parts of middle molecular weight dextran. The method comprises the steps that the dextran is dissolved by adding part of injection water, and stirring and filtering are conducted; the dexmedetomidine hydrochloride is taken, after the dexmedetomidine hydrochloride is dissolved by adding in citricacid-sodium citrate buffering liquid, the dexmedetomidine hydrochloride is added to a decarburized solution, and the injection water is additionally added to the full amount; the PH value is adjustedto 5.0-6.0, microfiltration is conducted, and the injection is obtained after freezing drying is conducted. The injection has the advantages of being good in resolubility, and high in stability and safety.
Owner:宁波蒙曼生物科技有限公司
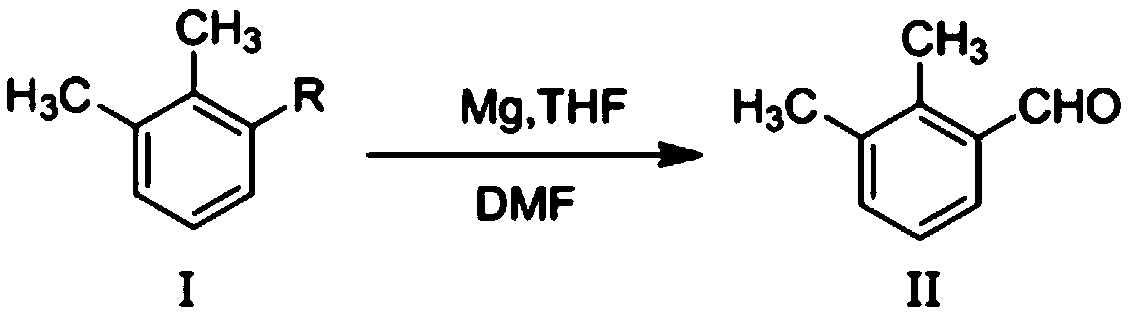
Method for preparing 2,3-dimethyl benzaldehyde
InactiveCN105503551ARich sourcesIndustrial applicabilityCarbonyl compound preparation by hydrolysisBenzaldehydeGrignard reagent
The invention discloses a method for preparing 2,3-dimethyl benzaldehyde. The method includes the steps that firstly, under the protection of nitrogen, a Grignard reagent is prepared from 2,3-dimethyl benzene halide; secondly, the Grignard reagent and N,N-dimethyl formamide are reacted; finally, the 2,3-dimethyl benzaldehyde is prepared through hydrolyzing, purifying and separating. The method is reasonable in design, mild in reaction condition, simple in technology and easy and convenient to operate; the obtained 2,3-dimethyl benzaldehyde is a key intermediate for synthesizing dexmedetomidine hydrochloride, the sources of the raw materials are rich, and industrial applicability is achieved.
Owner:SUOANKE SHANGHAI INVESTMENT CO LTD
Method for preparing dexmedetomidine hydrochloride for anesthesia and sedation during operation
The invention discloses a method for preparing dexmedetomidine hydrochloride for anesthesia and sedation during an operation. The method comprises the following steps: (a) stirring and reacting 2,3-dimethyl styrene and imidazole in ionic liquid to obtain 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole in the presence of an iron salt; (b) reacting the 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole with L-(+)-tartaric acid, and performing suction filtering to obtain dexmedetomidine tartrate; (c) stirring the dexmedetomidine tartrate in an aqueous solution of sodium hydroxide, performing dichloromethane extraction and concentration, and stirring in a saturated hydrochloric acid methanol solution to obtain the dexmedetomidine hydrochloride. According to the method, the ionic liquid is used for alkylation reaction of the imidazole under catalysis of the iron salt, so that the reaction time is effectively shortened, and high yield is achieved; fewer steps are required, and the cost is lower; conditions in each step are mild, use of a large amount of lewis acid is avoided, and easiness for industrial production is achieved.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
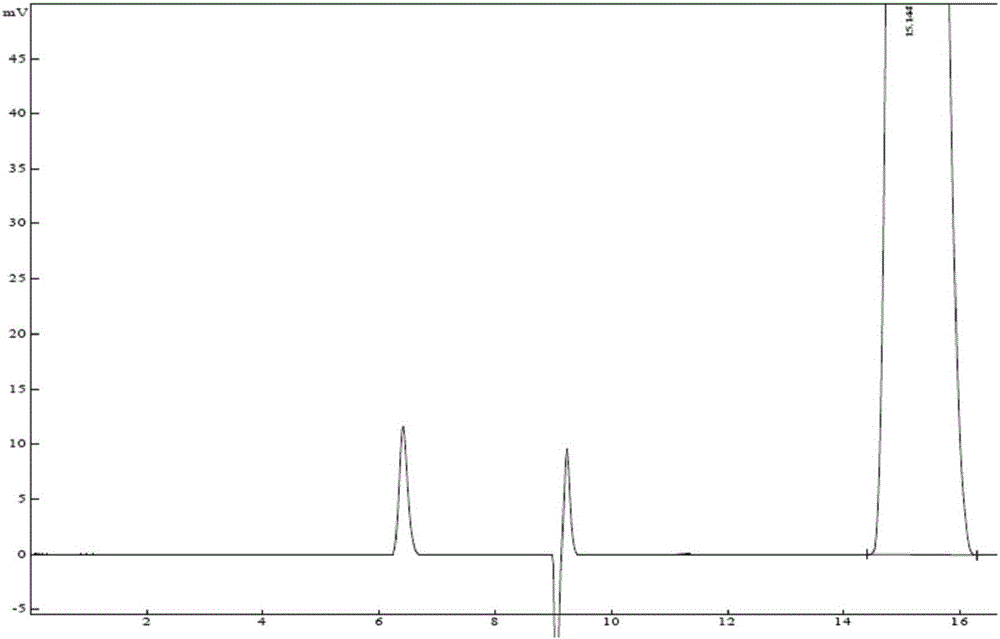
Method for detecting isomers of dexmedetomidine hydrochloride crude product
InactiveCN106442765AAccurate detectionEfficient separationComponent separationOrganic baseColumn temperature
The invention belongs to the technical field of drug detection, and particularly relates to a method for detecting isomers in a dexmedetomidine hydrochloride crude product. The detection method includes the specific step of conducting detection by high performance liquid chromatography, wherein a chromatographic column refers to a chiral column chromatography column or an N-lycopodine oxide M bonded silica gel column, flow velocity is 0.2-2.0 ml / min, detection wavelength is 180-380nm, column temperature is 0-40 DEG C, and mobile phase is n-hexane-ethyl alcohol-organic acid and / or organic base. Compared with the prior art, the method for detecting the isomers in the dexmedetomidine hydrochloride crude product has good specificity and high accuracy, a main peak and the isomers can be effectively separated, the isomers in the dexmedetomidine hydrochloride crude product can thus be accurately detected, and quality control of the product and establishment of a unified standard are well promoted.
Owner:CISEN PHARMA
Method for detecting bacterial endotoxin in dexmedetomidine hydrochloride by gel process
PendingCN111781365AEliminate interferenceReduce testing costsMaterial analysisBiochemistryMedetomidine
The invention relates to a method for detecting bacterial endotoxin in dexmedetomidine hydrochloride by a gel process. The method comprises the following steps of: preparing a dexmedetomidine hydrochloride solution by using dimethyl sulfoxide as a solvent, and diluting the prepared dexmedetomidine hydrochloride solution by using dimethyl sulfoxide and / or a diluent I and bacterial endotoxin inspection water until a concentration not lower than a minimum effective active concentration to prepare a test solution; and carrying out bacterial endotoxin detection by adopting a gel process. By adopting the detection method, the interference effect of dexmedetomidine hydrochloride on bacterial endotoxin can be eliminated, the dilution multiple is small, the detection result is accurate and reliable, the operation is simple and convenient, the detection cost is low, and compared with photometric detection, the operation is convenient and simple.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Preparation process of dexmedetomidine hydrochloride for ICU (intensive care unit) sedation and analgesia
The invention discloses a preparation process of dexmedetomidine hydrochloride for ICU (intensive care unit) sedation and analgesia. The preparation process includes the steps: first, stirring and reacting 1-(1-halogenated ethyl)-2, 3-dimethyl benzene and imidazole in ionic liquid under catalysis of ferric trichloride to obtain 4-[1-(2, 3-dimethyl phenyl) ethyl]-1H-imidazole; second, refluxing the 4-[1-(2, 3-dimethyl phenyl) ethyl]-1H-imidazole obtained in the first step and L-(+)-tartaric acid in absolute ethyl alcohol, and performing standing, cooling and suction filtration to obtain dexmedetomidine tartrate; third, stirring the dexmedetomidine tartrate in sodium hydroxide aqueous solution, extracting methylene dichloride, concentrating the solution, stirring the solution in saturated hydrochloric acid methanol solution to obtain the dexmedetomidine hydrochloride. The method is short in reaction time, high in yield, milder in condition and suitable for industrial popularization.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
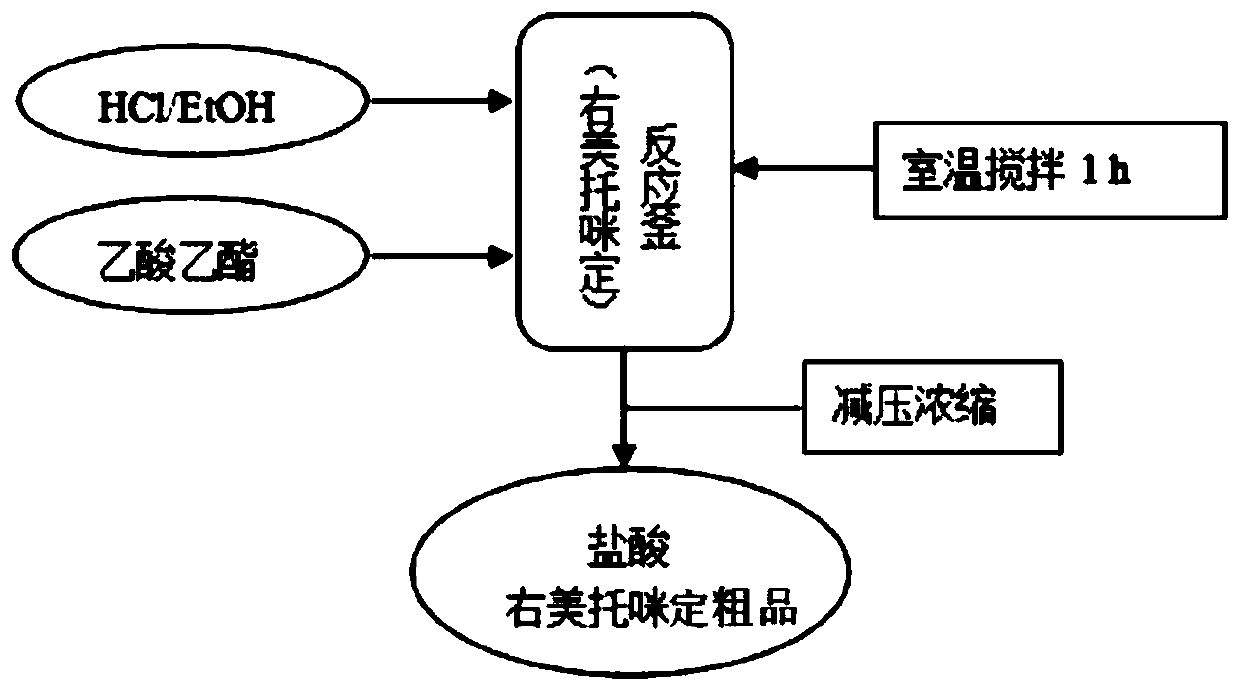
Preparation method of dexmedetomidine hydrochloride
InactiveCN111253316AEasy to separate and purifyEasy to routeOptically-active compound separationOrganic racemisationEthyl groupEthyl acetate
The invention discloses a preparation method of dexmedetomidine hydrochloride. The preparation method comprises the following steps: in a reaction solvent dichloromethane, carrying out a Friedel-Crafts reaction on 1-(1-chloroethyl)-2,3-dimethylbenzene and N-TMS imidazole under the action of lewis acid titanium tetrachloride to generate medetomidine; in a reaction solvent ethanol, resolving medetomidine by using L-(+)-tartaric acid to obtain medetomidine with a dextrorotation configuration; in a reaction solvent ethyl acetate, salifying dexmedetomidine in a hydrochloric acid solution to obtaina crude dexmedetomidine hydrochloride product; and finally, carrying out refining in a recrystallization mode to obtain a finished dexmedetomidine hydrochloride product. The preparation method has fewsteps, is simple and convenient to operate and is suitable for industrial production.
Owner:BOSEN BIO PHARMA SHANXI PROVINCE
Method for synthesizing dexmedetomidine hydrochloride intermediate
InactiveCN106588779AIncrease profitShort reaction timeOrganic chemistrySynthesis methodsTriflic acid
The invention discloses a method for synthesizing a dexmedetomidine hydrochloride intermediate. The synthesis method comprises the step that 1-(1-trifluoromethanesulfonate)ethyl-2,3-dimethylbenzene and imidazole are subjected to stirring reacting in ionic liquid in the presence of alkali and [1,1'-bis(diphenylphosphine)ferrocene]palladium dichloride to obtain the dexmedetomidine hydrochloride intermediate 4-[1-(2,3-dimethylbenzene)ethyl]-1H-imidazole. The method is short in reacting time and high in product production efficiency and yield and has the good selectivity on a target (S)-isomer.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com