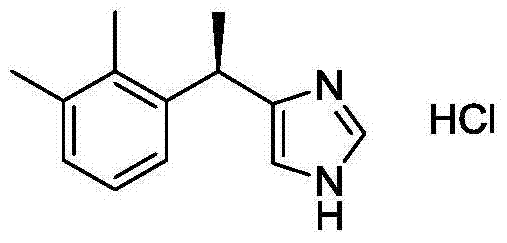
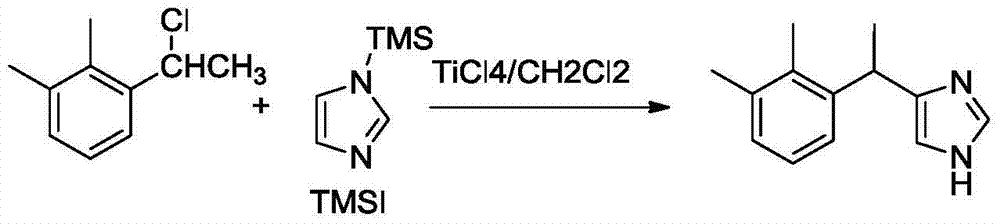
New method for preparing dexmedetomidine hydrochloride
A technology for dexmedetomidine hydrochloride and dexmedetomidine, which is applied in the field of pharmaceutical chemical synthesis and can solve the problem of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Preparation of 1-(1-chloroethyl)-2,3-dimethylbenzene (2)
[0012] In a 1000mL three-neck flask, add 200mL of tetrahydrofuran (THF) and 12g (0.5mol) of magnesium strips, heat to reflux, add dropwise 150mL of a THF solution of 92.5g (0.5mol) of 2,3-dimethylbromobenzene, and reflux for 1 hour; Cool to room temperature, dropwise add acetaldehyde 36mL (0.64mol) / THF100mL, continue to reflux for 1h, evaporate THF under reduced pressure, slowly add NH 4 Cl aqueous solution (25g NH 4 C1+80mL water) and ethyl acetate 300mL. Static stratification, with anhydrous Na 2 S0 4 Dry the organic phase, filter, evaporate the solvent, and then distill under reduced pressure to collect fractions at 115-118°C / 300Pa. Add anhydrous ZnCl 2 200g and 300mL of concentrated HCl, fully dissolved and added 150g (1mol) of the above fraction, and stirred vigorously for 4h. Layered, anhydrous CaCl 2 The organic phase was dried, filtered, the filtrate was evaporated to remove the solvent, and disti...
Embodiment 2
[0014] Preparation of medetomidine (1)
[0015] In a 1000mL three-necked flask, add 0.2mol of Lewis acid and 200mL of solvent, dropwise add TMSI14.7mL (0.1mol) and 40mL of solvent under ice bath, and continue to stir for 30min; slowly add compound 216.9g (0.1mol) and 40mL of solvent, control The temperature of the external bath was continued to stir for 1 h; then continued to stir at room temperature for 15 h. Under ice bath (below 10°C), add 400 mL of water dropwise, stir well to make it completely hydrolyzed, transfer the reaction solution to a separatory funnel and let it stand for stratification; Use solvent 200mL×3 to extract in the funnel; after combining the organic phases, wash 100mL×3 times with water, and use Na 2 SO 4 After drying, the solvent was evaporated to obtain a light yellow viscous substance, which was dissolved in 2 mol / L HC aqueous solution, and 5 mol / L NaOH was added dropwise to produce a flocculent white precipitate, which was filtered and dried to ob...
Embodiment 3
[0017] Preparation of dexmedetomidine
[0018] In a 500 mL three-neck flask, compound 1 (17.5 g, 87 mmol) was added to 120 mL of isopropanol-water (volume ratio 1:1) solution, heated to 80° C. to completely dissolve the solid. At this temperature, add D-DTTA (18.5g, 48mmol) into the reaction system to dissolve it. After stirring for 30 minutes, stop heating, cool to 15°C, and continue stirring for 3 hours. A large amount of white precipitates gradually precipitate out of the system. Filter it quickly and use Wash with cold isopropanol several times, filter with suction to obtain a white solid, dissolve it in 100mL of water, slowly add saturated Na 2 CO 3 , the solution until the pH value of the system was 7-8, and fully stirred for 1h, extracted with ethyl acetate (50mL×3), combined the organic phases, anhydrous Na 2 SO 4 After drying, the ethyl acetate was distilled off under reduced pressure to obtain a white solid (S)-1 (7.7 g), with a yield of 44%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com