Dexmedetomidine hydrochloride injection and preparation process thereof
A technology of dexmedetomidine hydrochloride and injection, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve the problem of accelerated foreign matter, increased foreign matter, and increased visible foreign matter in drugs, etc. problem, to achieve the effect of low cost, stable quality, and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
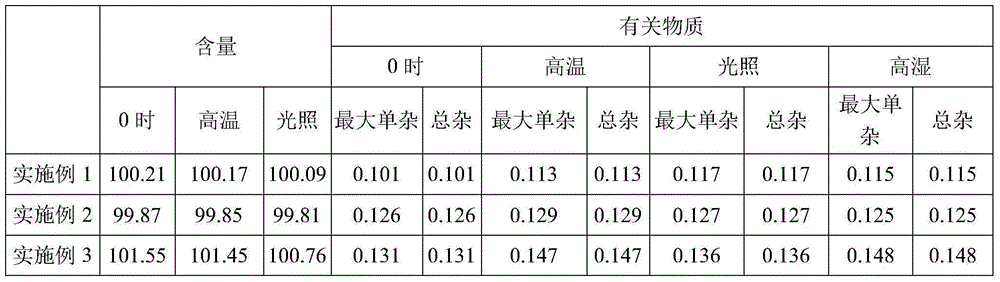
[0015] Raw materials: 0.2g of dexmedetomidine hydrochloride (calculated as dexmedetomidine), 18g of sodium chloride, 0.08g of sodium tartrate, add water for injection to 2000ml, and make 1000 sticks.
[0016] Preparation method: 1) take 95% of the prescription amount of water for injection, boil it and lower the temperature to 60°C; 2) turn on the stirrer, add the prescription amount of sodium chloride, stir for 5 minutes to dissolve it; add dexmedetomidine hydrochloride , stir for 10 minutes to make it completely dissolve and mix evenly; 3) then cool down to 30°C, add the prescribed amount of sodium tartrate, stir to make it completely dissolved, add active carbon with a main material amount of 3% (w / w) and stir for 15 minutes ; 4) Add water for injection to the full amount, continue to stir and circulate for 15 minutes, filter with a 0.5 μm titanium rod and a 0.22 μm microporous filter, take a sample at the sampling port to detect the content and pH of the liquid medicine, an...
Embodiment 2
[0018] Raw materials: dexmedetomidine hydrochloride (calculated as dexmedetomidine) 0.2g, sodium chloride 18g, edetate calcium sodium 0.08g, dihydroconiferyl alcohol γ-O-α-L-rhamnoside 0.02 g, add water for injection to 2000ml, and make 1000 sticks.
[0019] Preparation method: 1) take water for injection with 95% of the prescription amount, boil it and lower the temperature to 70°C; 2) turn on the stirrer, add the prescription amount of sodium chloride, stir for 5 minutes to dissolve it; add dexmedetomidine hydrochloride , stirred for 10 minutes to completely dissolve and mix evenly; 3) then lower the temperature to 50°C, add the prescribed amount of calcium sodium edetate and dihydroconiferyl alcohol γ-O-α-L-rhamnoside, and stir to make it Completely dissolve, add activated carbon with 3% (w / w) main ingredient, stir and adsorb for 15 minutes; 4) add water for injection to the full amount, continue to stir and circulate for 15 minutes, and filter with 0.5μm titanium rod and 0...
Embodiment 3
[0021] Raw materials: dexmedetomidine hydrochloride (calculated as dexmedetomidine) 0.2g, sodium chloride 18g, edetate calcium sodium 0.08g, add water for injection to 2000ml, and make 1000 sticks.
[0022] Preparation method: 1) take water for injection with 95% of the prescription amount, boil it and lower the temperature to 70°C; 2) turn on the stirrer, add the prescription amount of sodium chloride, stir for 5 minutes to dissolve it; add dexmedetomidine hydrochloride , stir for 10 minutes to make it completely dissolve and mix evenly; 3) then cool down to 50°C, add the prescribed amount of edetate calcium sodium, stir to make it completely dissolve, add 3% (w / w) of active carbon as the main ingredient and stir Adsorb for 15 minutes; 4) Add water for injection to the full amount, continue to stir and circulate for 15 minutes, filter with a 0.5 μm titanium rod and a 0.22 μm microporous filter, take a sample at the sampling port to detect the content and pH value of the drug s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com