Nasal drops used for anesthesia and preparation method of nasal drops
A technology of nasal drops and solutions, which is applied in the field of anesthesia nasal drops and its preparation, can solve the problems of increased risk of aspiration, easy crystallization, and life-threatening problems, and achieves simple and easy preparation process and small increase of impurities , Good compliance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of nasal drop for anesthesia is prepared according to the following steps:
[0026] Element
Dosage (parts by weight)
1 serving
Ketamine hydrochloride
720 copies
glucose
2100 copies
50 servings
350 servings
glycerin
25 copies
polyethylene glycol 200
40 servings
36 servings
11000 copies
[0027] Preparation process:
[0028] 1. Concentrated preparation: add 1 / 5 of the prescription amount of purified water to the batching tank, add the prescription amount of dexmedetomidine hydrochloride, ketamine hydrochloride, glucose, ethylparaben, sodium thiosulfate, set the speed at 60~ 80 rev / min, stir to dissolve solution 1, set aside; take another batching tank, add 2 / 5 of the prescription amount of purified water, add the prescription amount of glycerin, polyethyle...
Embodiment 2
[0065] A nasal drop for anesthesia, prepared as follows:
[0066] Element
Dosage (parts by weight)
1 serving
Ketamine hydrochloride
600 copies
glucose
1800 copies
30 copies
300 copies
glycerin
20 copies
polyethylene glycol 200
30 copies
22 servings
purified water
9000 copies
[0067] Preparation process: according to the preparation process of Example 1, it was prepared.
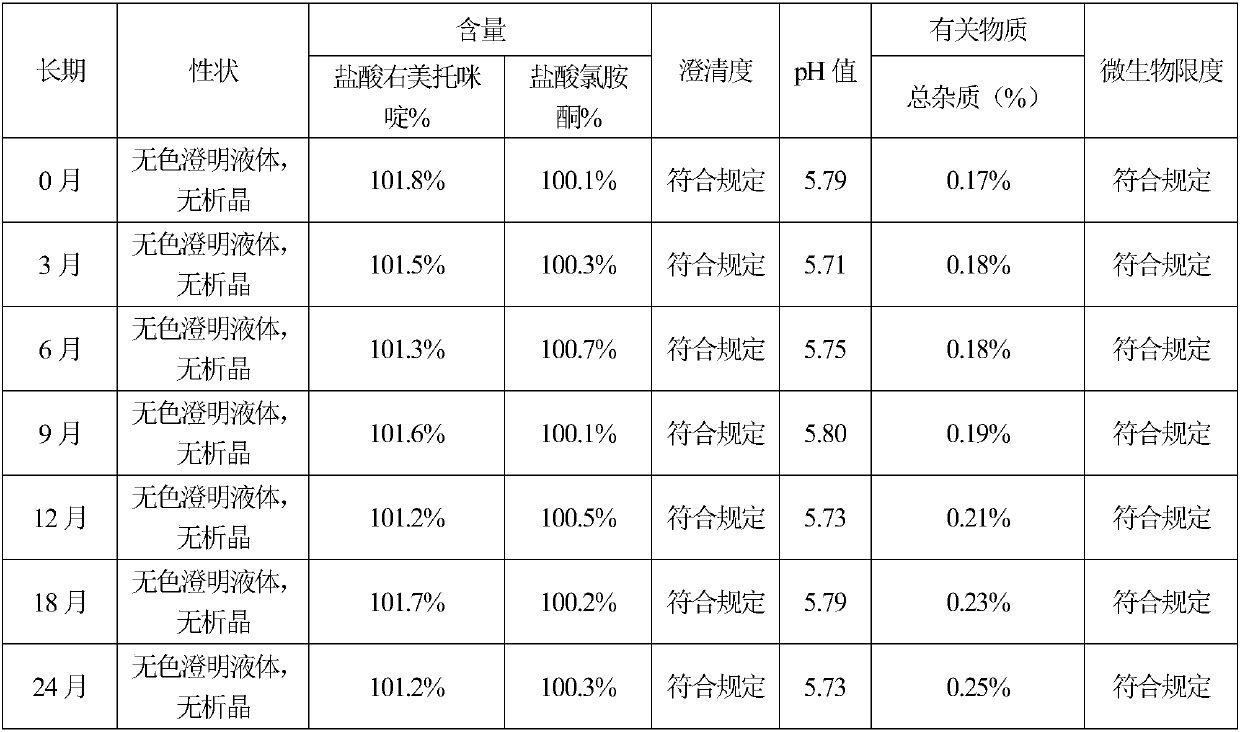
[0068] According to the test method of Example 1, the results of the sample stability test in Example 2 show that the quality of the sample is stable in the accelerated six months, and the quality is stable in the long-term 24 months, so the validity period of this product is at least 24 months; the influence of the types of auxiliary materials and the preparation process on the increase of impurities The test re...
Embodiment 3
[0070] A nasal drop for anesthesia, prepared as follows:
[0071] Element
Dosage (parts by weight)
1 serving
Ketamine hydrochloride
800 copies
glucose
2300 copies
60 servings
400 copies
glycerin
29 servings
polyethylene glycol 200
50 servings
51 copies
purified water
12000 copies
[0072] Preparation process: according to the preparation process of Example 1, it was prepared.
[0073] According to the test method of Example 1, the results of the sample stability test in Example 3 show that the quality of the sample is stable in the accelerated six months, and the quality is stable in the long-term 24 months, so the validity period of this product is at least 24 months; the influence of the types of auxiliary materials and the preparation process on the increase of impurities The te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com