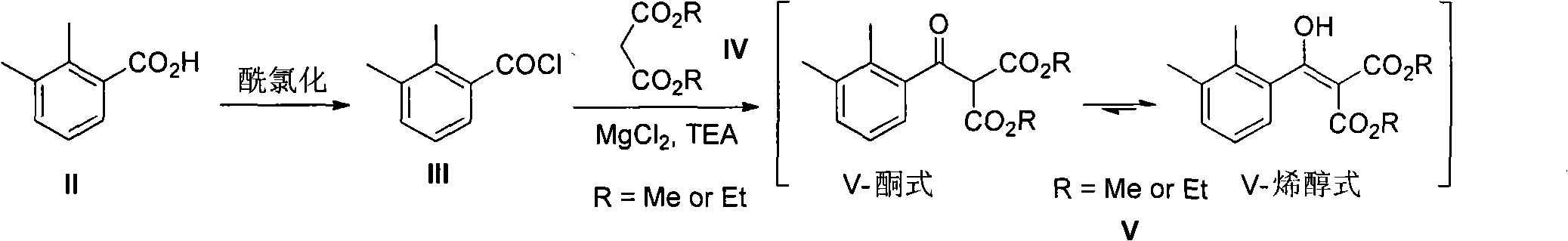
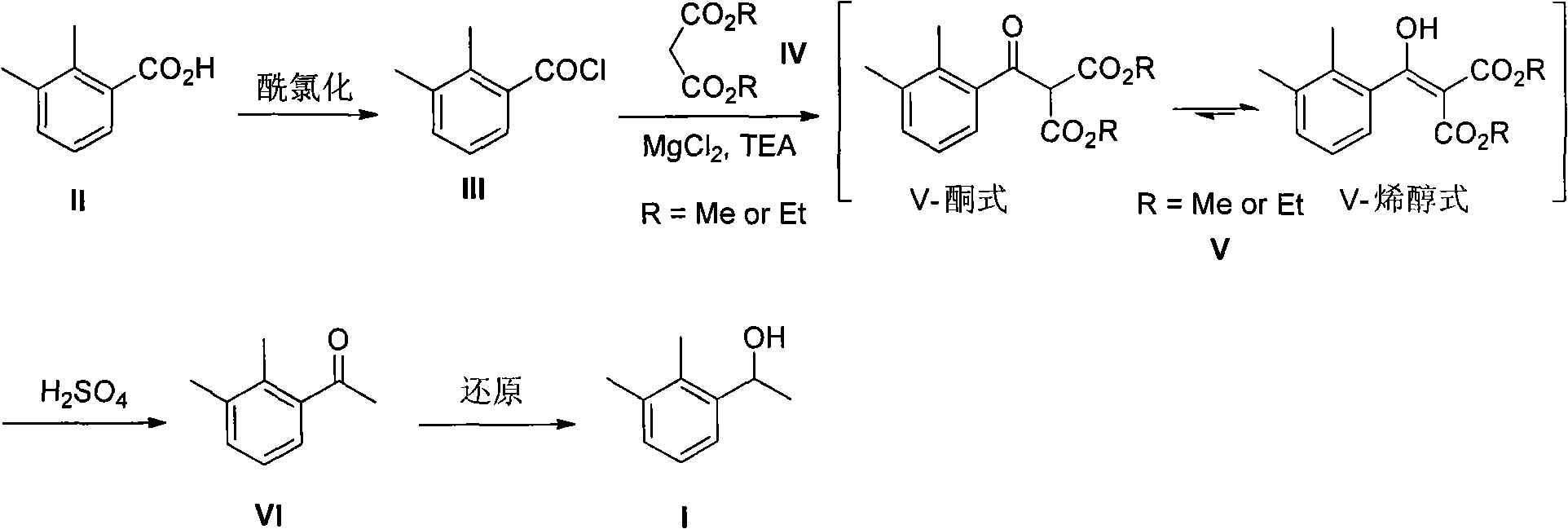
2-(2,3-dimethyl phenyl) diester malonate, preparation method thereof, and application thereof
A technology of dimethylphenyl and malonic acid diester is applied in the field of preparation of dexmedetomidine hydrochloride intermediate 1-ethanol, and can solve the problems of harsh reaction conditions, inflammable and explosive, inconvenient operation and the like, and achieves the Mild conditions, easy to scale production, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve II (8.25g) in 80mL of dichloromethane, add 8.90g of thionyl chloride, drop a few drops of DMF to initiate the reaction, stir at room temperature overnight, evaporate the solvent to obtain acid chloride III; 11.71g of diethyl malonate , 14.73g of triethylamine and 3.50g of anhydrous magnesium chloride were put into 70mL of ethyl acetate and stirred at room temperature for 45 minutes. After cooling slightly to 10°C, the above-prepared acid chloride III was slowly added dropwise to the system. Stir for 50 minutes to end the reaction, wash with 10% dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine successively, dry the organic layer through anhydrous magnesium sulfate, and obtain 18.60 g of the crude product of V after removing the solvent; in order to obtain the analysis of V With the sample, 3.40g of it was purified by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) to obtain 2.81g of pure V, which was a c...
Embodiment 2
[0022] Dissolve II (8.25g) in 80mL of dichloromethane, add 10.20g of thionyl chloride, drop a few drops of DMF to initiate the reaction, stir at room temperature overnight, evaporate the solvent to obtain acid chloride III; add 9.64g of dimethyl malonate , 14.73g of triethylamine and 3.50g of anhydrous magnesium chloride were put into 70mL of ethyl acetate and stirred at room temperature for 45 minutes. After cooling slightly to 10°C, the above-prepared acid chloride III was slowly added dropwise to the system. Stir for 50 minutes to end the reaction, wash with 10% dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine successively, dry the organic layer through anhydrous magnesium sulfate, and obtain 15.46 g of the crude product of V after removing the solvent; in order to obtain the analysis of V With the sample, 3.0 g of it was purified by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20: 1) to obtain 2.63 g of pure V, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com