Preparation process of dexmedetomidine hydrochloride for ICU (intensive care unit) sedation and analgesia
A technology of dexmedetomidine hydrochloride and dexmedetomidine, which is applied in the field of preparation technology of dexmedetomidine hydrochloride, can solve the problems of harsh synthesis conditions and unsatisfactory yield of intermediate medetomidine, and achieve Ease of industrial production, shortened reaction time, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Preparation of ionic liquids
[0029] ①[Omim]BF 4 (l-octyl-3-methylimidazolium tetrafluoroboric acid)
[0030] Add 82.1g N-methylimidazole and 163.5g 1-chlorooctane in the 500mL three-neck flask that stirrer, reflux condenser and thermometer are housed, and add 80mL cyclohexane and toluene respectively as reaction intermediary, control the The temperature was 75°C, and after stirring for 48 hours, the solvent in the upper layer and unreacted raw materials were removed by pouring. The oily liquid in the lower layer was washed with ethyl acetate 4 times while hot (20 mL each time), and the liquid in the lower layer was transferred to a one-necked bottle, and depressurized. Part of the solvent and unreacted raw materials in the raw materials were removed by rotary evaporation. After the rotary evaporation was completed, it was transferred to a vacuum drying oven at a temperature of 70° C. and dried in vacuum for 36 hours to obtain the intermediate product chlorinated l-o...
Embodiment 1
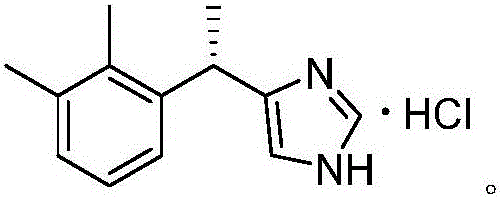
[0038] Preparation of 4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole
[0039] FeCl 3 3.2g (20mmol), 1-(1-chloroethyl)-2,3-dimethylbenzene 16.8g (100mmol), imidazole 7.5g (110mmol) and ionic liquid ([omim]BF 6 ) 35ml into the reaction flask, stirred and reacted at 75°C for 0.5 hours, after monitoring the reaction, poured into water, extracted with dichloromethane, washed the organic phase three times with water, dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure, and weighed petroleum ether. Crystallization gave 17.0 g of 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole, with a yield of 84.8%.
[0040] 1 HNMR (400MHz, CDCl 3 ): δ7.32(s,1H),7.06-6.95(m,3H),6.69(s,1H),4.40(q,J=21.2,7.2,1H),2.29(s,3H),2.31(s ,3H), 1.59 (d, J=7.6,3H).
Embodiment 2
[0042] Preparation of 4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole
[0043] FeCl 3 ·6H 2 O 8.1g (30mmol), 1-(1-chloroethyl)-2,3-dimethylbenzene 16.8g (100mmol), imidazole 8.2g (120mmol) and ionic liquid [Bmim]BF 6 Add 45ml into the reaction flask, stir and react at 80°C for 0.5 hours, monitor the completion of the reaction, pour into water, extract with dichloromethane, wash the organic phase three times with water, dry the organic phase with anhydrous sodium sulfate, concentrate under reduced pressure, and recrystallize petroleum ether 16.9 g of 4-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole was obtained with a yield of 84.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com