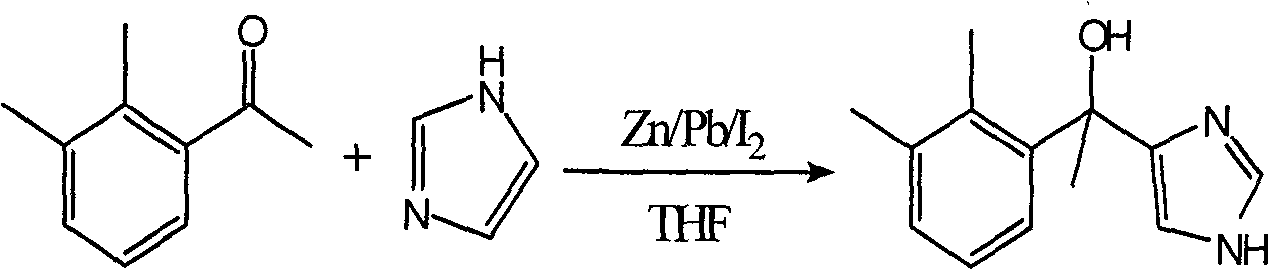Preparation of dexmedetomidine hydrochloride key intermediate
A technology for dexmedetomidine hydrochloride and an intermediate, which is applied in the field of preparing 1--1-ethanol, can solve the problems of high cost, many reaction steps, complicated operations and the like, and achieves fast reaction speed, easy control of conditions, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1 Preparation of 1-(2,3-dimethylphenyl)-1-(1H-imidazol-4-yl)ethanol
[0024] Add 97.5g of zinc powder (1.5mol), 3.9g of lead powder (0.018mol) and 150ml of dry THF into a 1L three-neck flask, heat to reflux, and stir for 3h. Allow to cool naturally. Under the ice-salt bath, 150ml of THF solution in which 76.2g of iodine (0.3mol) was dissolved was slowly added dropwise to the three-necked flask, and the temperature was controlled below 30°C during the dropwise addition. After dropping, stir and react at about 0°C for 30 minutes. Continue to cool down to -10°C, slowly add dropwise a solution of 76.8g (0.39mol) of 4-iodoimidazole dissolved in 210ml of dry THF, control the temperature at -10°C to -5°C, and keep stirring for 30min after dropping. After that, continue to slowly add 44.4g (0.3mol) of 2,3-dimethylacetophenone dropwise at -10°C ~ -5°C. After the drop is complete, the temperature is naturally raised to room temperature, and the reaction is stirred for 2...
example 2
[0025] Example 2 Purification of 1-(2,3-dimethylphenyl)-1-(1H-imidazol-4-yl)ethanol
[0026] Add 18g of the obtained crude product into 50ml of ethanol to dissolve, add 100ml of water and mix evenly, crystallize at -5°C to 0°C for 12 hours, a large amount of white crystals precipitate, filter with suction, wash the filter cake twice with 10ml of water, the obtained white crystals are in After drying under reduced pressure at 50°C for 12 hours, 16.8 g (93.3%) of the product was obtained. The purity by PHLC was 98.37%, and the mp was 134°C-137°C.
example 3
[0027] Example 3 Purification of 1-(2,3-dimethylphenyl)-1-(1H-imidazol-4-yl)ethanol
[0028] Take 17.6g of the crude product, add 50ml of ethanol to dissolve it, add 50ml of water, stir and mix evenly, the resulting solution is crystallized at -5°C to 0°C for 12 hours, a large amount of white crystals are precipitated, suction filtered, the filter cake is washed twice with 10ml of water, and The resulting white crystals were vacuum-dried at 50°C for 12 hours to obtain 16.1 g (91.5%) of the product, with a purity of 99.23% by HPLC and an mp of 137°C-140°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com