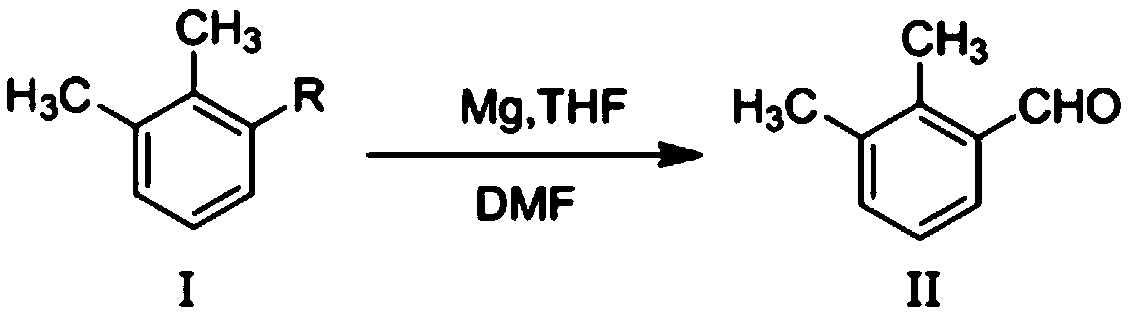
Method for preparing 2,3-dimethyl benzaldehyde
A technology of dimethylbenzaldehyde and dimethylhalogenated benzene, which is applied in the chemical field, can solve the problems of butyllithium use safety risks, high price, unsatisfactory reaction yield, and no large-scale commercial sources, etc., reaching Low price, easy operation, reasonable design effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Under nitrogen protection, 24 grams of magnesium chips (1.0mol) and 160 milliliters of tetrahydrofuran were added to a reaction flask, and a mixture of 18.5 grams of 2,3-dimethylbromobenzene (0.1mol) and 100 milliliters of tetrahydrofuran was added dropwise under stirring at room temperature. solution to initiate the reaction, and then continue to dropwise add a mixed solution of 166.5 grams of 2,3-dimethylbromobenzene (0.9mol) and 1000 milliliters of tetrahydrofuran. Cool the resulting Grignard reagent with an ice bath, add dropwise a mixture of 73 grams of N,N-dimethylformamide (1.0mol) and 300 ml of tetrahydrofuran, control the reaction temperature not higher than 30 degrees, and drop it at room temperature The reaction was continued for 2 hours. After the reaction, add 1000 ml of saturated ammonium chloride solution for hydrolysis for 1 hour, then let stand to separate the organic phase, extract the water phase once with ethyl acetate, combine the organic phases, wa...
Embodiment 2
[0013] Under nitrogen protection, 26.4 grams of magnesium chips (1.1mol) and 160 milliliters of tetrahydrofuran were added in a reaction flask, and a mixture of 18.5 grams of 2,3-dimethylbromobenzene (0.1mol) and 100 milliliters of tetrahydrofuran was added dropwise under stirring at room temperature. liquid to initiate the reaction, and then continue to dropwise add a mixed solution of 166.5 grams of 2,3-dimethylbromobenzene (0.9mol) and 1000 milliliters of tetrahydrofuran, and after the addition is complete, reflux for 1 hour. Cool the resulting Grignard reagent with an ice bath, add dropwise a mixture of 73 grams of N,N-dimethylformamide (1.0mol) and 300 ml of tetrahydrofuran, control the reaction temperature not higher than 20 degrees, and drop it at room temperature The reaction was continued for 2 hours. After the reaction, add 1000 ml of saturated ammonium chloride solution for hydrolysis for 1 hour, then let stand to separate the organic phase, extract the water phase ...
Embodiment 3
[0015] Under nitrogen protection, 24 grams of magnesium chips (1mol) and 160 milliliters of tetrahydrofuran were added to a reaction flask, and a mixture of 18.5 grams of 2,3-dimethylbromobenzene (0.1 mol) and 100 milliliters of tetrahydrofuran was added dropwise with stirring at room temperature , Initiate the reaction, then continue to dropwise add a mixed solution of 166.5 grams of 2,3-dimethylbromobenzene (0.9mol) and 1000 milliliters of tetrahydrofuran, after the addition is complete, reflux for 1 hour. Cool the resulting Grignard reagent with an ice bath, add dropwise a mixture of 73 grams of N,N-dimethylformamide (2.0 mol) and 300 ml of tetrahydrofuran, control the reaction temperature not higher than 20 degrees, and drop it at room temperature The reaction was continued for 2 hours. After the reaction, add 1000 ml of saturated ammonium chloride solution for hydrolysis for 1 hour, then let stand to separate the organic phase, extract the water phase once with ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com