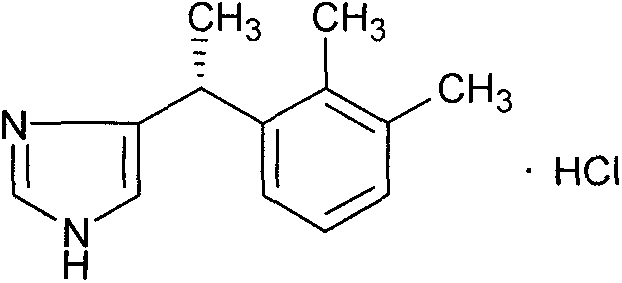
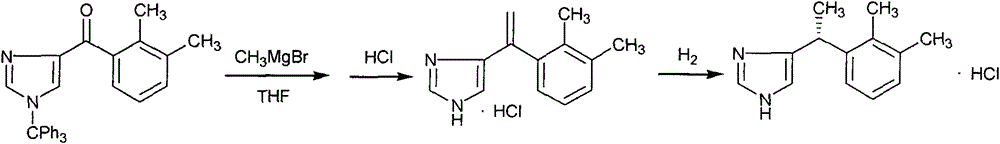
New method for preparing dexmedetomidine hydrochloride key intermediate
A technology for dexmedetomidine hydrochloride and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of uneconomical reactions, expensive raw materials, cumbersome processing, etc., and achieves the advantages of repeated and expanded scale preparation and easy post-processing , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11- 3
[0029] The preparation of embodiment 11-trityl-4-(N-methyl-N-methoxy) imidazole carboxamide
[0030]
[0031] Dissolve 100mL of tetrahydrofuran and 25g of 1-trityl imidazole-4-carboxylic acid at room temperature, stir and dissolve, add 19g of CDMT in batches, after the addition, add 29g of triethylamine dropwise to control the internal temperature not exceeding 30°C, react for 1-2 hours, TLC shows the raw material After the reaction is complete, add 21 g of DMHH, react overnight at room temperature, TLC shows that the reaction is complete, filter, and concentrate the filtrate to dryness under reduced pressure; add 300 mL of dichloromethane and stir to dissolve, wash with water, saturated aqueous sodium bicarbonate solution, 1N dilute hydrochloric acid, and water successively, and anhydrous Dry over sodium sulfate, filter, concentrate to dryness, add 50mL of petroleum ether (60-90) and stir for 30min, filter, and dry the filter cake at 50°C under normal pressure for 6h to obt...
Embodiment 22
[0032] Embodiment 22, the preparation of 3-dimethylphenylmagnesium bromide tetrahydrofuran solution
[0033]
[0034] Put 100mL of tetrahydrofurfural and 2.7g of magnesium flakes in a 1L three-necked flask, protect it with nitrogen, stir at room temperature, add 18.5g of 2,3-dimethylbromobenzene dropwise, and continue stirring at room temperature overnight to obtain 1mol / L A solution of 2,3-dimethylphenylmagnesium bromide in tetrahydrofuran.
Embodiment 3
[0035] Preparation of Example 34-(2,3-dimethylbenzoyl)-1-(trityl)imidazole
[0036]
[0037] Put 120mL of tetrahydrofuran and 20g of intermediate formula II in a 1L three-necked flask, protect it under nitrogen, stir and dissolve at room temperature, add 200mL of 1mol / L 2,3-dimethylphenylmagnesium bromide tetrahydrofuran solution dropwise, drop The reaction was completed for 1-2 hours, monitored by TLC. After the reaction was completed, 200 mL of saturated ammonium chloride solution was slowly added dropwise, stirred at room temperature for 1 hour, allowed to stand and separated, and the organic phase was washed once with saturated brine. The organic phase was dried over anhydrous sodium sulfate, pumped Filter, wash the filter cake with an appropriate amount of tetrahydrofuran, concentrate the filtrate under reduced pressure at 40°C to dryness to obtain 22g of the crude product, add 100mL of absolute ethanol to reflux for 30min, cool to 0°C, stir and crystallize for 1h, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com