Method of measuring content of related substance in dexmedetomidine hydrochloride active ingredient
A technology for dexmedetomidine hydrochloride and related substances, which is applied in the field of drug analysis and can solve problems to be improved and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The selection of embodiment 1 chromatographic conditions
[0058] wavelength selection
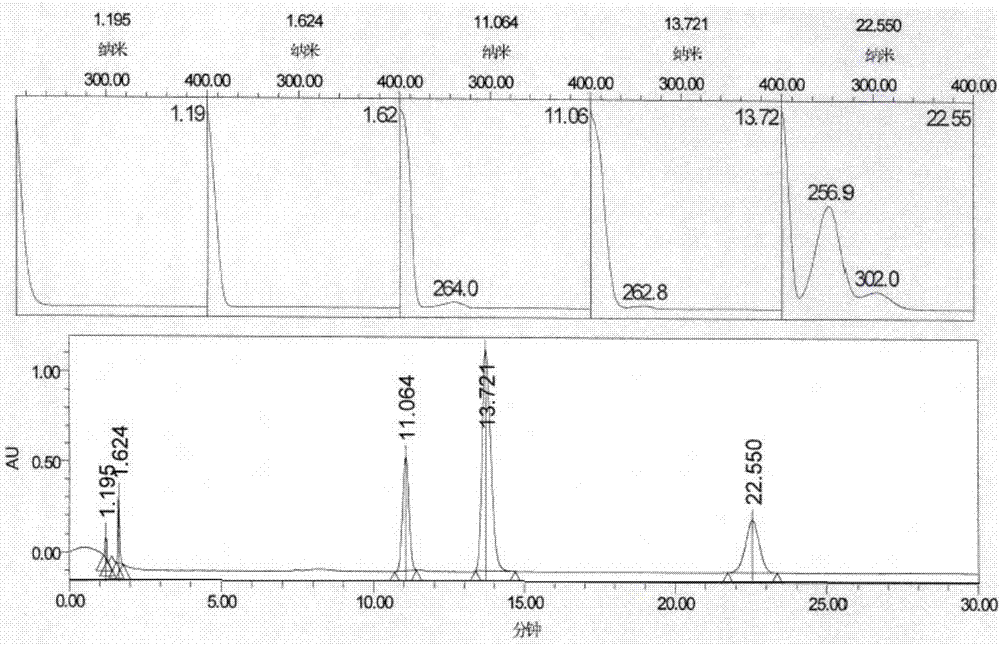
[0059] Starting material 1, intermediate 2, intermediate 3, and impurity B in the dexmedetomidine hydrochloride crude drug are mixed and dissolved in water to form a mixed solution containing 0.5 mg / ml of each substance, sample injection, and use DAD (diode array detection device, which can also be expressed as PDA) for full-wavelength scanning, the chromatogram results are as follows figure 1 shown. figure 1 The results show that scanning the PDA chromatogram of each substance finds that all substances have terminal absorption peaks, and then select a wavelength with relatively large main peaks and impurity peaks, so the inventor selects a lower wavelength of 214nm.
[0060] pH selection
[0061] In the present invention, the inventor uses 0.03mol / L dipotassium hydrogen phosphate solution (phosphoric acid adjusts pH to 7.0) and 0.05mol / L potassium dihydrogen phosphate solution (...
Embodiment 2
[0084] Embodiment 2 specificity experiment
[0085] The verification process of the specificity experiment is as follows:
[0086] ① The finished product and each starting material and intermediate are injected separately to obtain a chromatogram; and after mixing, inject a sample to obtain a chromatogram;
[0087] ② Degrade the finished product under severe conditions such as acid, alkali, oxidation, pyrolysis, and light;
[0088] The acceptable detection criteria for the verification results of the specificity experiment are as follows:
[0089] ① The existence of each starting material and intermediate does not interfere with the detection of the main component;
[0090] ②The separation between the main peak and the impurity peak should be greater than 1.5, and the minimum separation between the impurity peaks should be greater than 1.5;
[0091] ③ The purity angle value of the main peak and the main impurities should be less than the purity threshold value (use PDA to s...
Embodiment 3
[0104] Embodiment 3 durability experiment
[0105] Verification process of durability test
[0106] Investigate mobile phase flow rate variation ± 10%, column temperature variation ± 5 ℃, mobile phase pH value variation and the variation of chromatographic column, to measure need testing solution (concentration of need testing solution is 0.5mg / ml, injection volume is 20ul) chromatographic changes in chromatographic behavior.
[0107] Acceptable criteria for the results of the durability test are as follows:
[0108] (1) The main peak tailing factor should be ≤ 2.0,
[0109] (2) The impurity peak and other component peaks must achieve baseline separation,
[0110] (3) The RSD of impurity content data under each condition should be ≤10.0%,
[0111] (4) The absolute value of the impurity content is within 0.1%.
[0112] The test results of the durability test are shown in Table 3-1.
[0113] Table 3-1:
[0114]
[0115] As can be seen from Table 3-1, the tailing factor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com