Method for detecting (R)-etiracetam from medicine
A detection method and detection condition technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of chromatographic column drop, trouble, and reduce the quality control standard limit of levetiracetam raw materials, so as to achieve no tailing, Improve the quality of medicines and detect the peak shape of the spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0065] Example 2 Analytical method validation
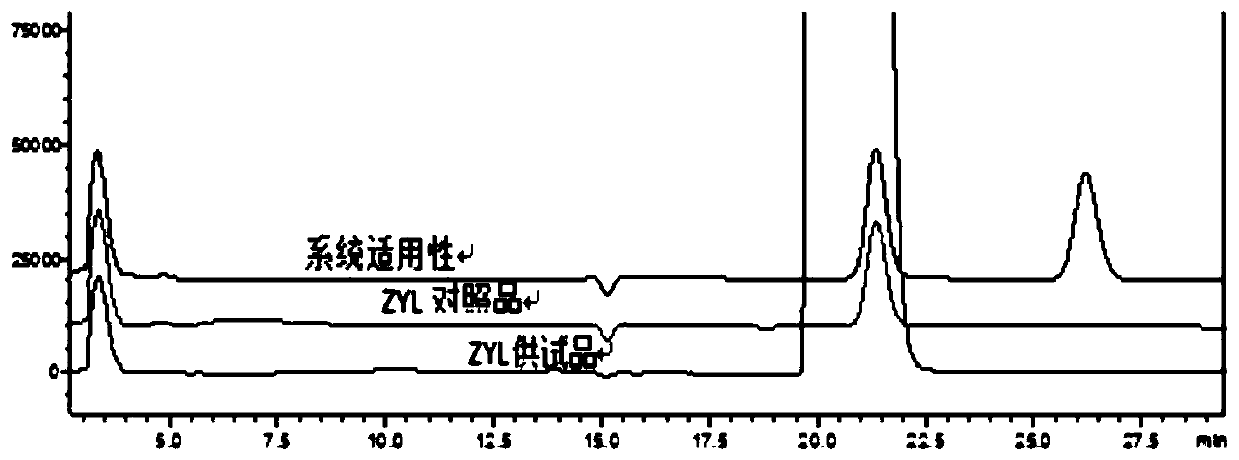
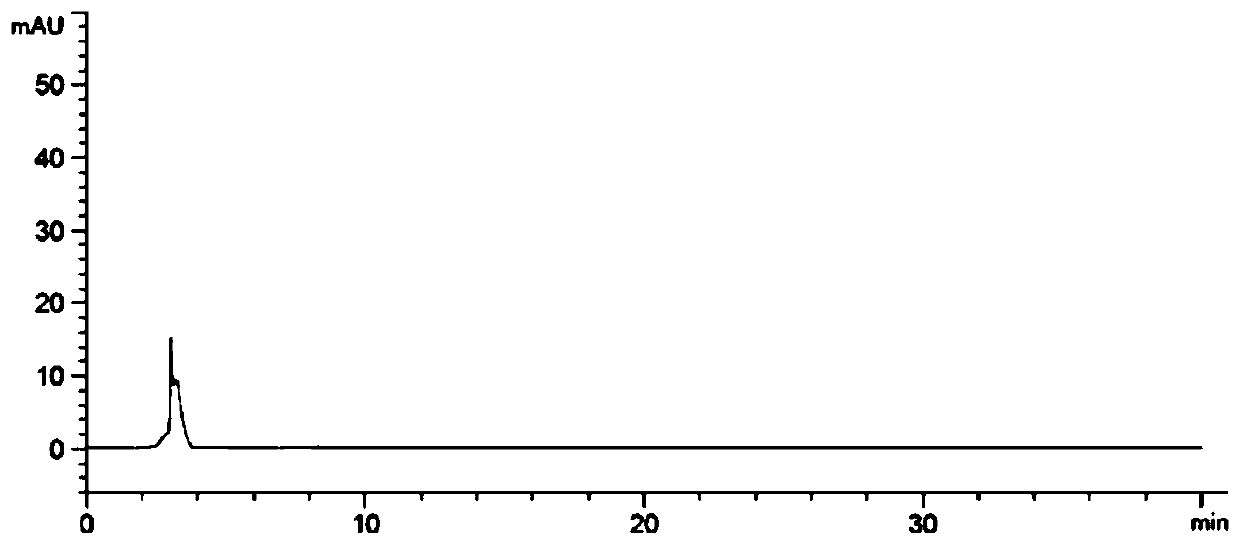
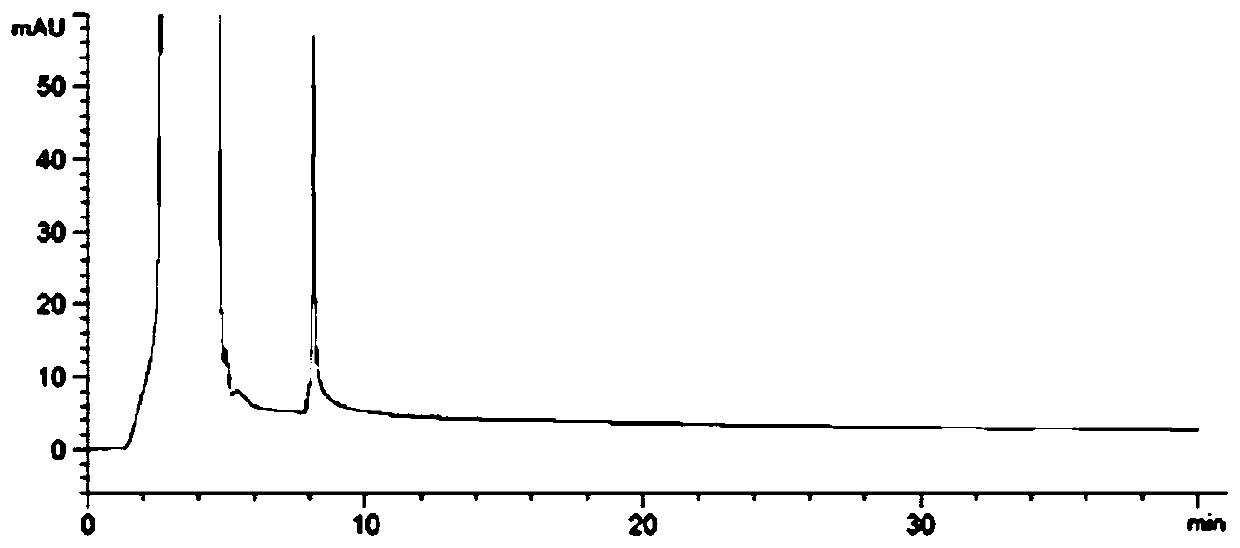
[0066] The invention preliminarily establishes the chromatographic conditions of the R-isomer analysis method, and the test results show that the above method can effectively separate the R-isomer from the main drug.
[0067] The specific verification results are shown in Table 4:
[0068] Table 4 Summary of validation of analytical methods for R-isomer of levetiracetam injection
[0069]
[0070]
[0071]
[0072] The results show that the system applicability and specificity, detection limit and quantification limit, linearity and range, precision, accuracy and durability of the method of the present invention's detection method all meet the requirements, proving that the detection method of the present invention is suitable for levothyrocytosis Detection of the R-isomer of Piracetam.
[0073] Further compare and illustrate technical effect of the present invention by control test below:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com