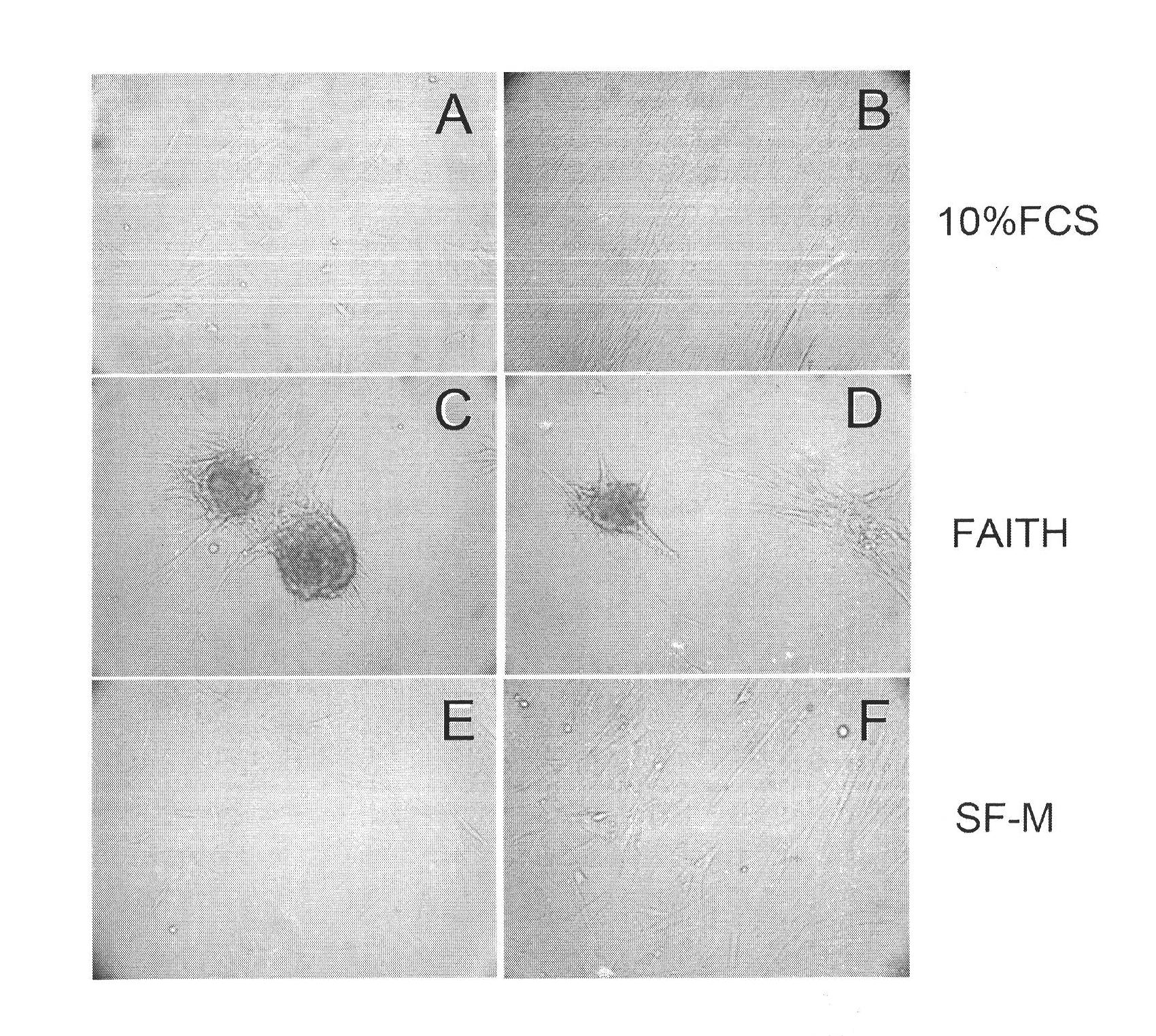
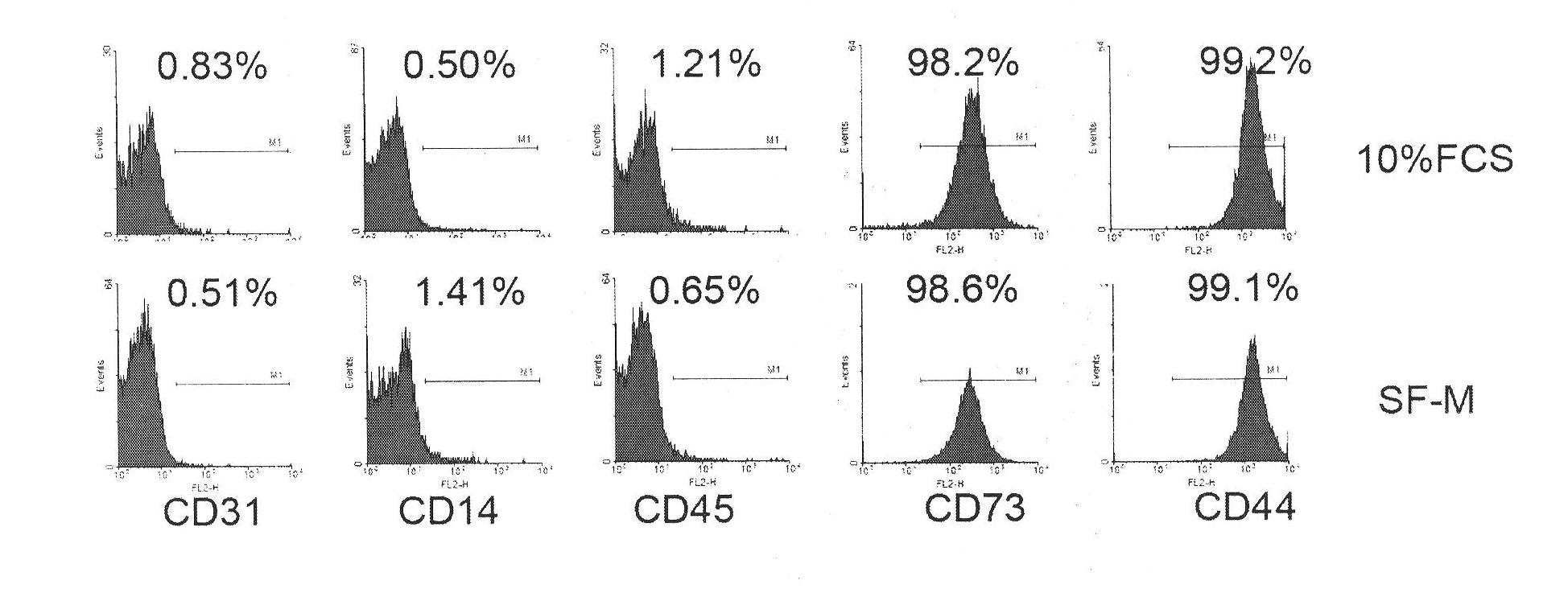
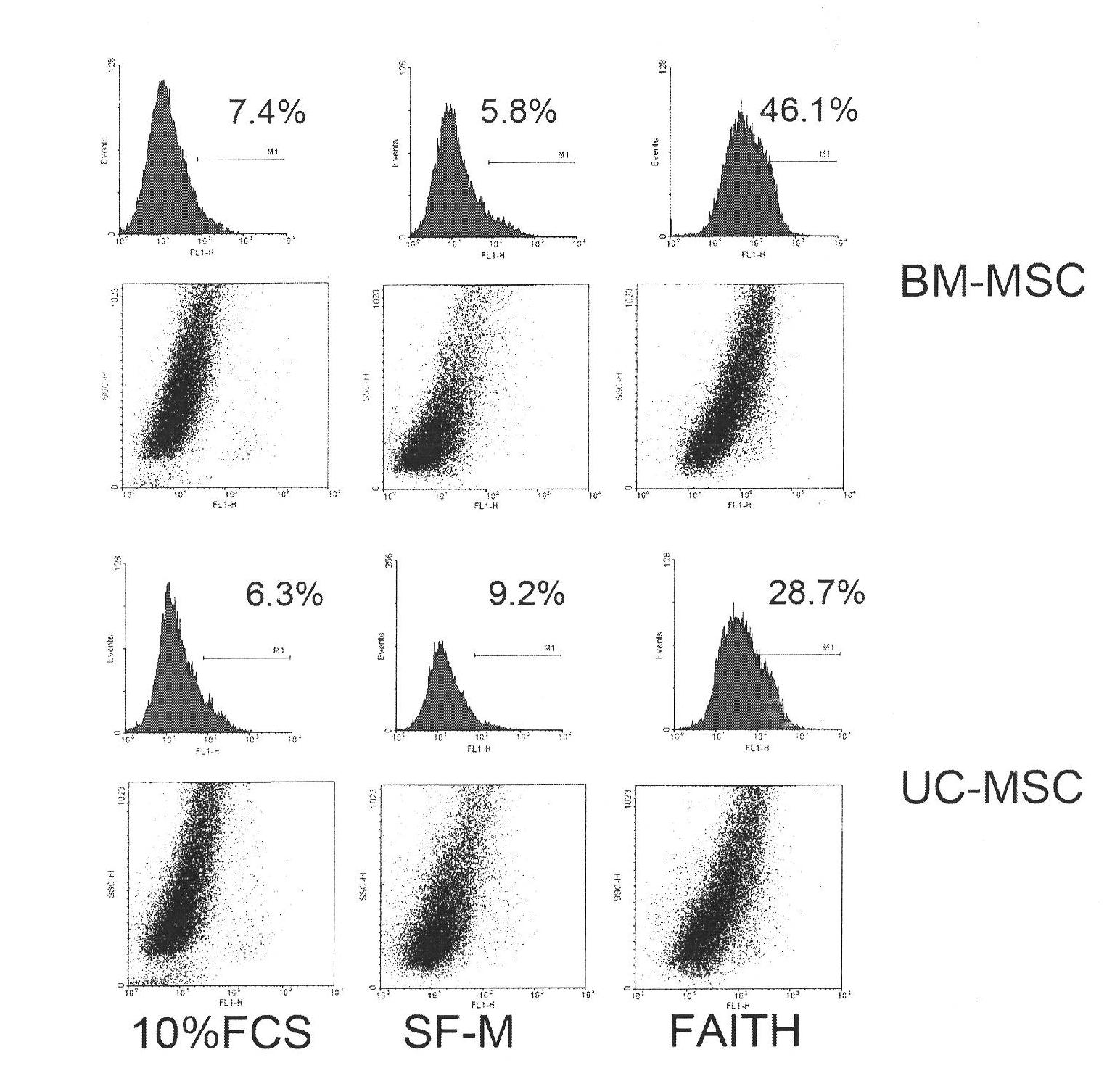
Culture medium for culturing mesenchymal stem cells
A technology of mesenchymal stem cells and medium, applied in the field of medium for culturing mesenchymal stem cells, to avoid batch differences, clear chemical composition, and broad application prospects.
Inactive Publication Date: 2011-03-09
INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
View PDF0 Cites 81 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Therefore, it can be considered that there may be many uncertain factors in the stability of MSC culture and the maintenance of stem cell pluripotency with serum substitutes such as bFGF-ITSE and hydrocortisone.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a culture medium for culturing mesenchymal stem cells. The culture medium comprises improved minimum medium IMDM, cell growth factor combination, insulin, trace elements, lipid substances, vitamin substances, hormone substances, protein molecules, amino acid substances and other compounds. The culture medium provided by the invention does not contain serum or plasma (comprising animal or human serum or plasma), thus, when the system is used for culturing the mesenchymal stem cells, zoonosis caused by the animal serum or plasma in the cell therapy process can be avoided, and the risk of human immunity reaction which is possibly caused in the cell therapy process can be avoided. The culture medium has specific chemical constituent, thus the possibility of different cultured mesenchymal stem cell properties caused by the difference of culture systems can be avoided. The culture medium is not only suitable for culturing the mesenchymal stem cells of human marrow and umbilical cord resources but also suitable for culturing the mesenchymal stem cells of other tissues or other animals, and has the advantage of wide application range.
Description
technical field [0001] The invention belongs to the culture medium formula in the field of cell and tissue culture, and relates to a serum-free culture medium formula with clear chemical components. The culture medium is especially suitable for culturing human mesenchymal stem cells in vitro. Background technique [0002] Mesenchymal stem cells, whose English name is mesenchymal stem cells (MSC), also have other names, such as bone marrow stromal cells, adipose stem cells, etc. -Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006;8(4):315-7 ). In the bone marrow, MSCs belong to non-hematopoietic stem cells, have the ability to differentiate into osteoblasts, chondrocytes, and adipocytes, and have immune regulation and hematopoietic support functions. Recent studies have shown that MSCs are also pr...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C12N5/0775
Inventor 郭子宽吕君吴祖泽王立生靳继德常菁
Owner INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com