Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Alternative strategy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for treating tumors and cancerous tissues
InactiveUS20050214268A1Improve bioavailabilityBiocideGenetic material ingredientsAbnormal tissue growthApoptosis
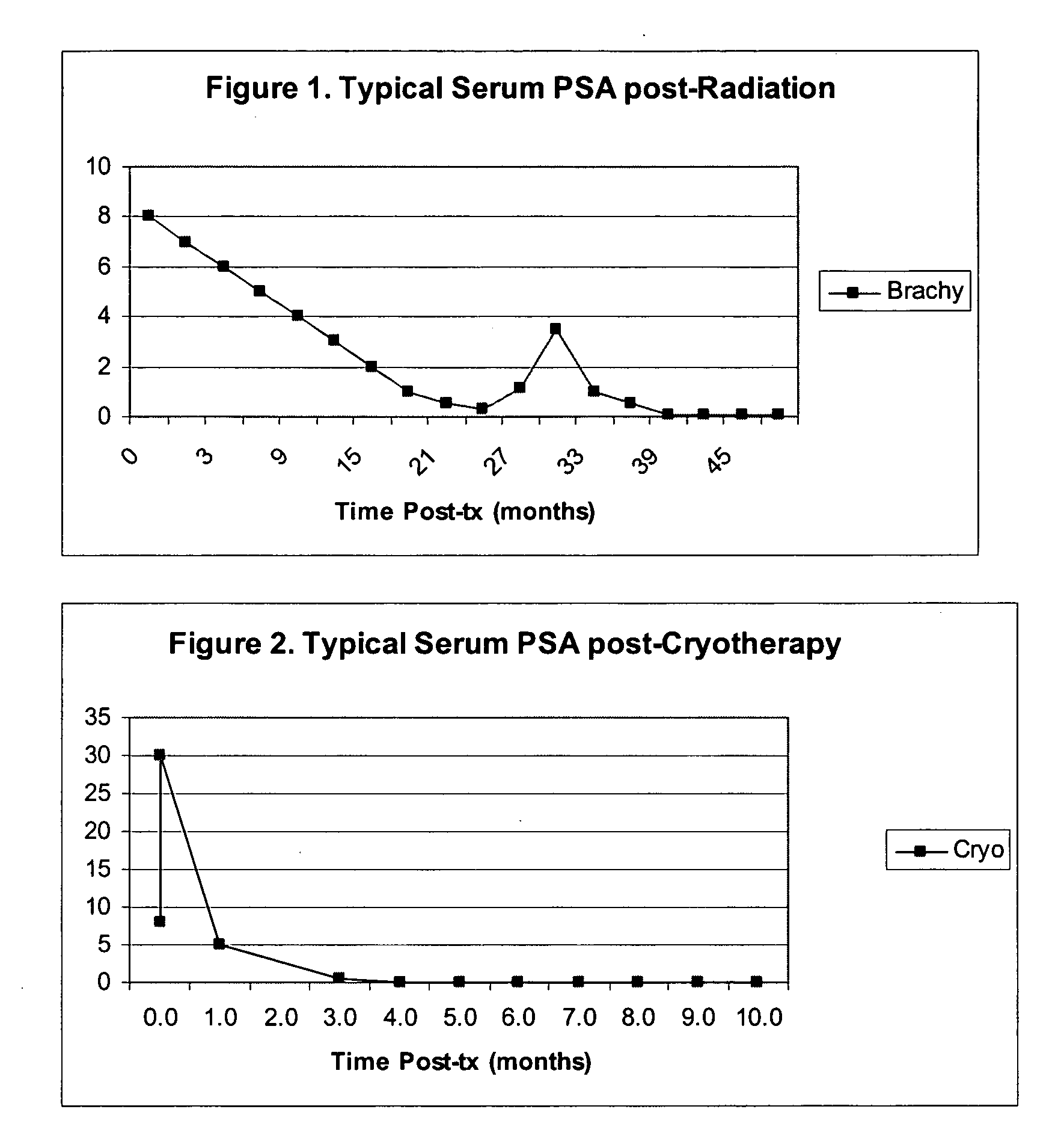
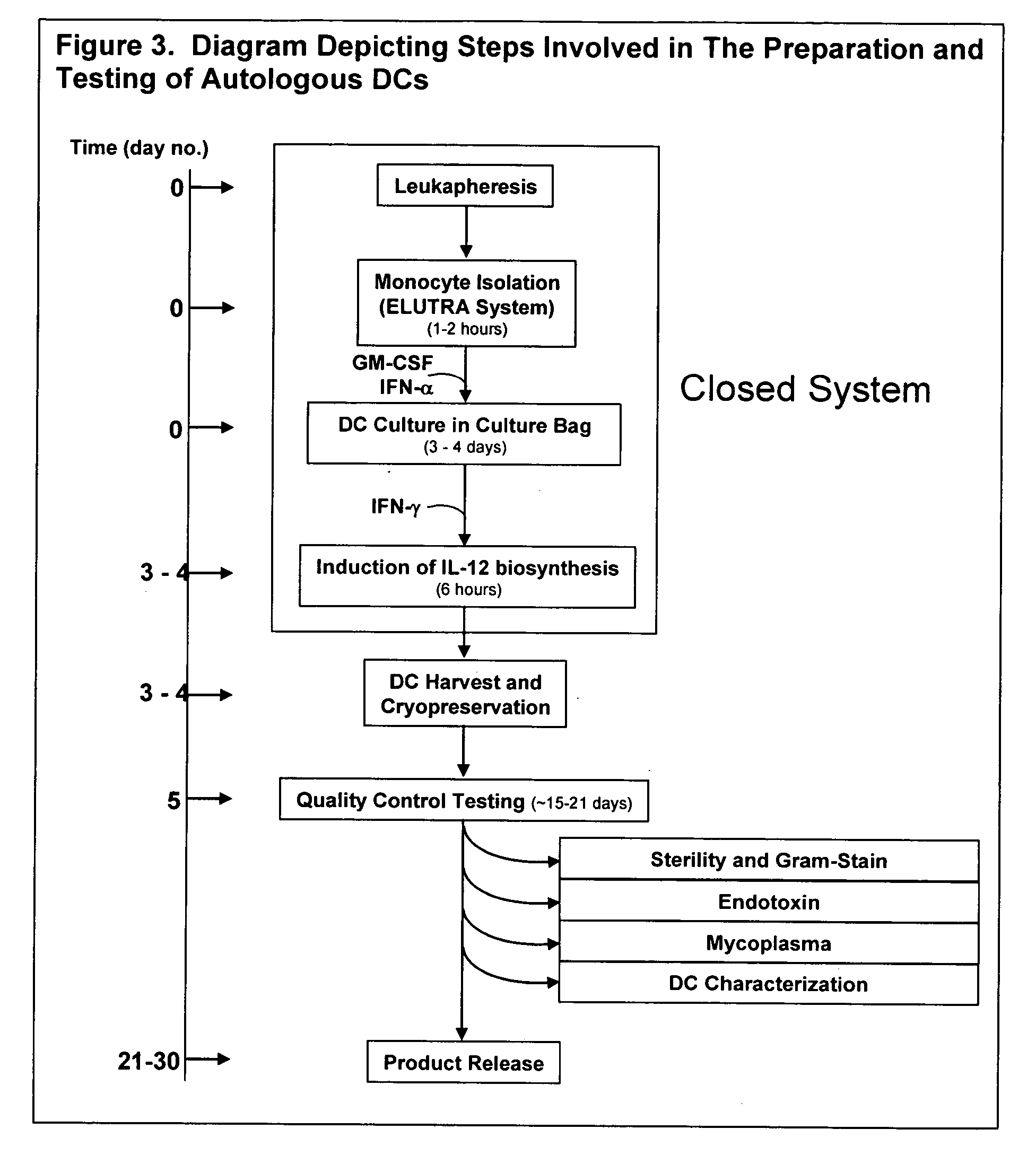
The invention disclosed herein relates generally to immunotherapy and, more specifically, to therapeutic methods for treating tumors and cancerous tissues by first inducing necrosis or apoptosis (e.g., cryotherapy, chemotherapy, radiation therapy, ultrasound therapy, or a combination thereof applied against at least a portion of the tumor or cancerous tissue), and then delivering one or more se doses of antigen presenting cells (e.g., autologous dendritic cells) intratumourally or proximate to the tumor or cancerous tissue, but only after a selected period of time sufficient for the bioavailablity of liberated cancer-specific antigens (monitored over the selected period of time) resulting from the necrosis or apoptosis to be at or near a maximum value. The present invention provides an alternative strategy to the ex vivo loading of target antigen to antigen presenting cells such as, for example, enriched autologous dendritic cells for purposes of enhancing an immune response.
Owner:SANGRETECH BIOMEDICAL
Apparatus and method for accelerated exhaust system component heating
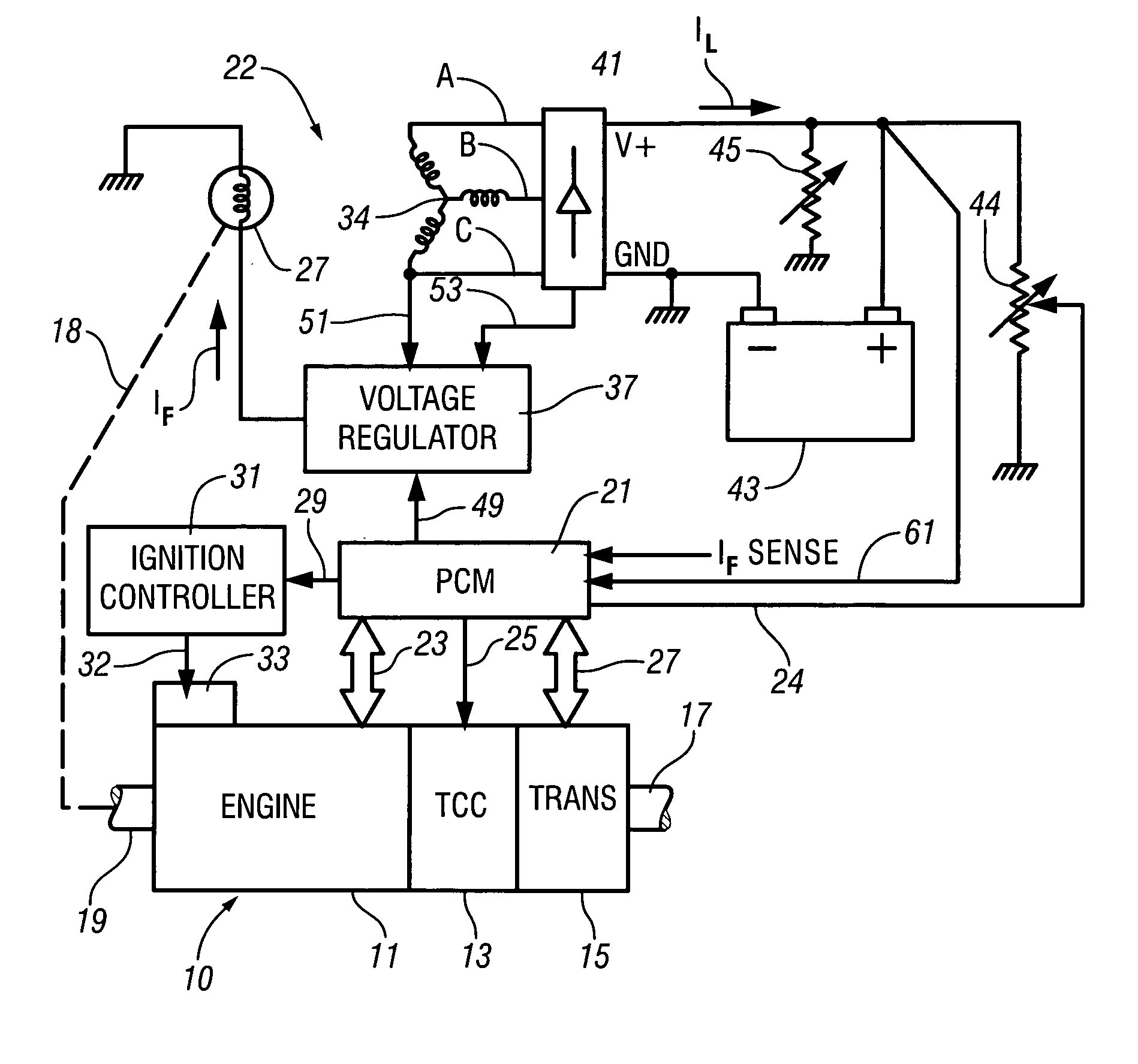
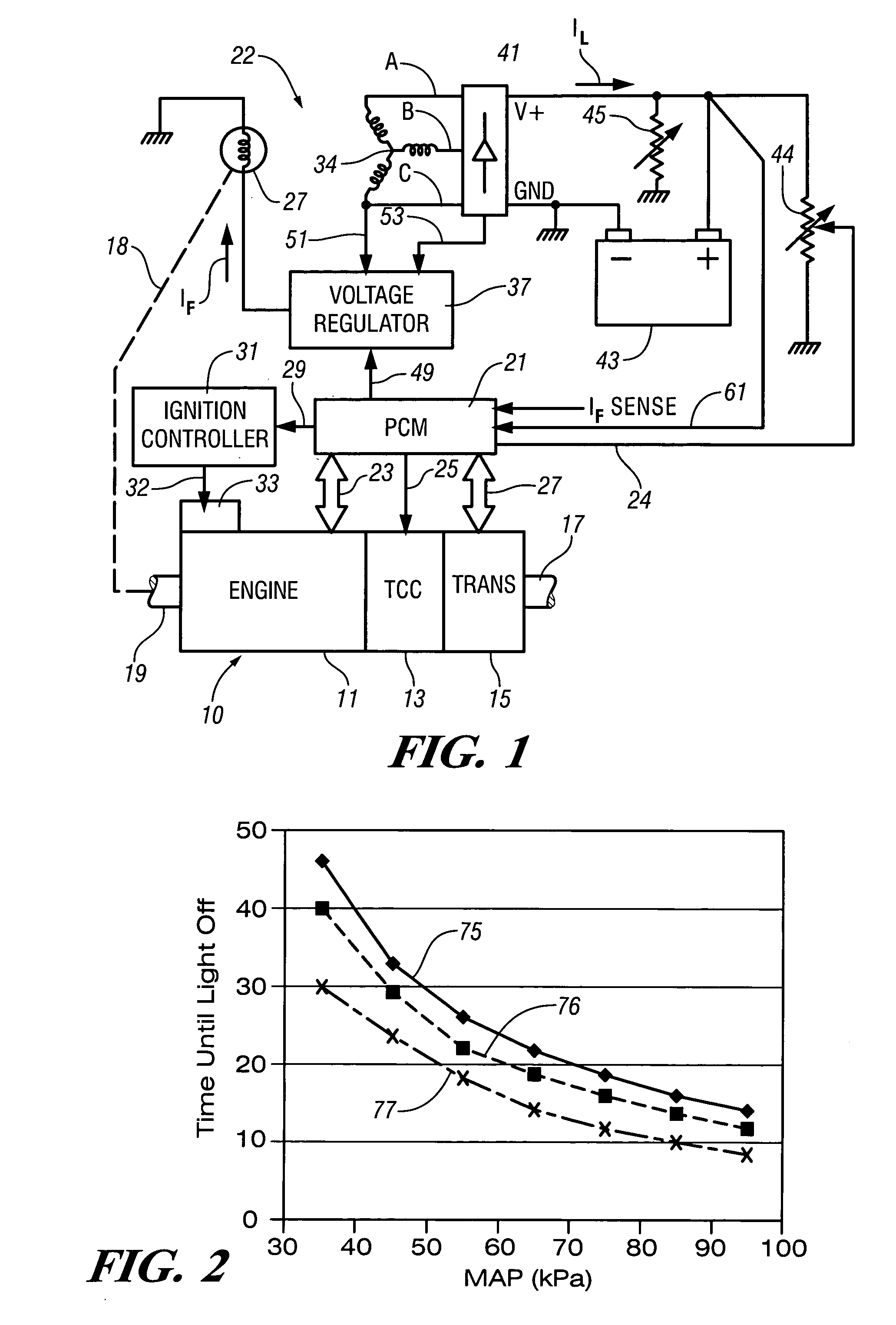
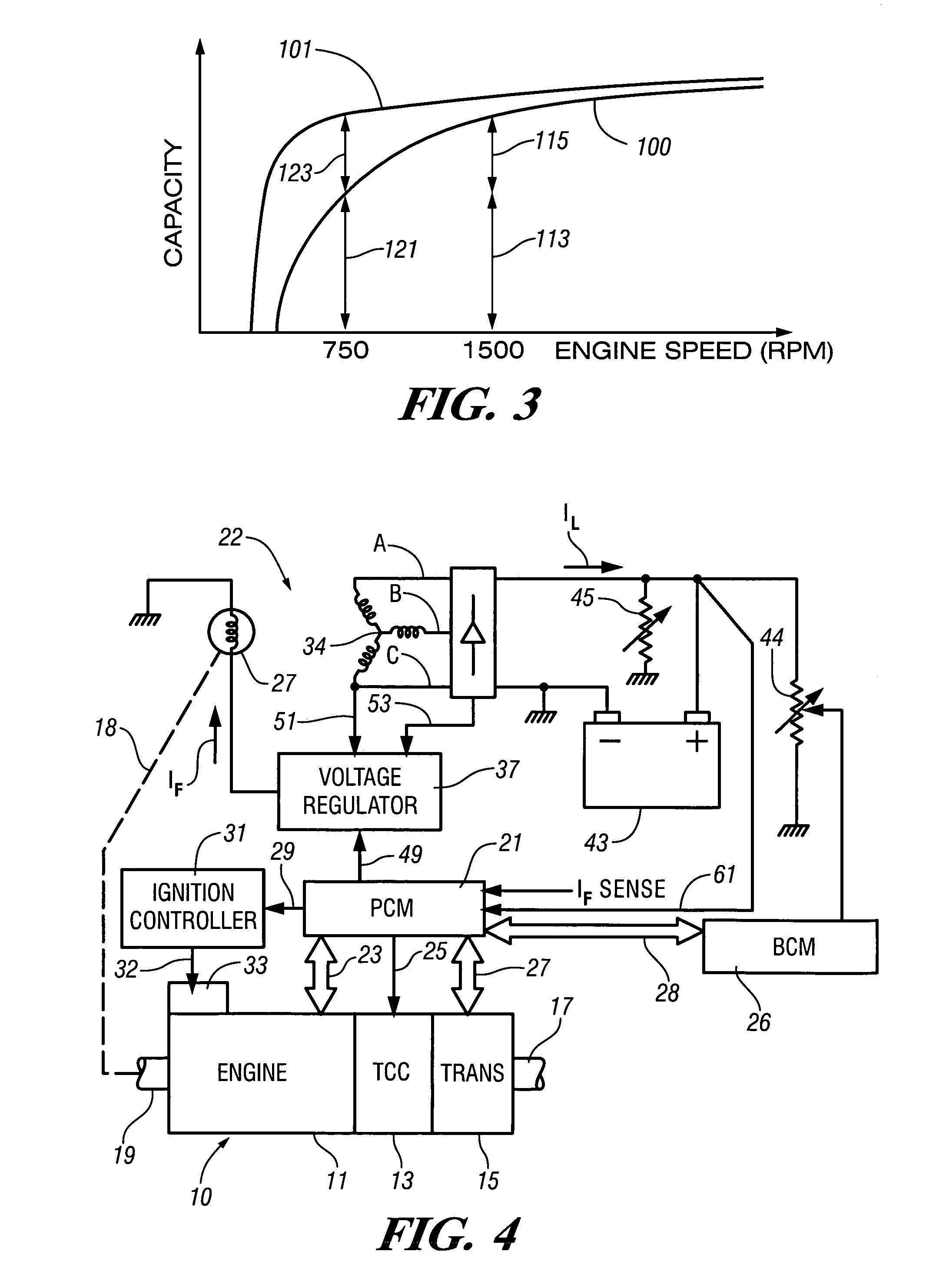
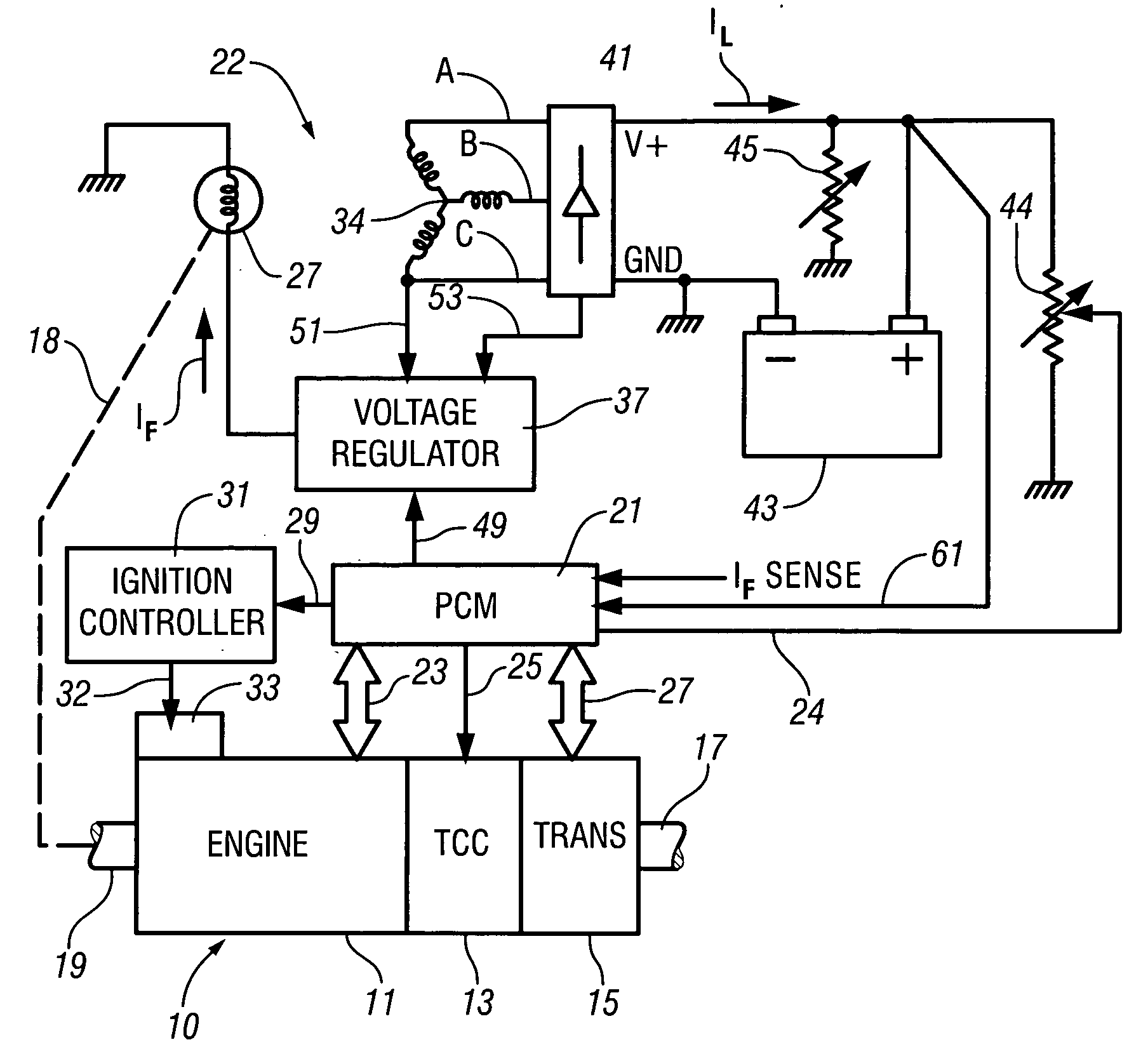
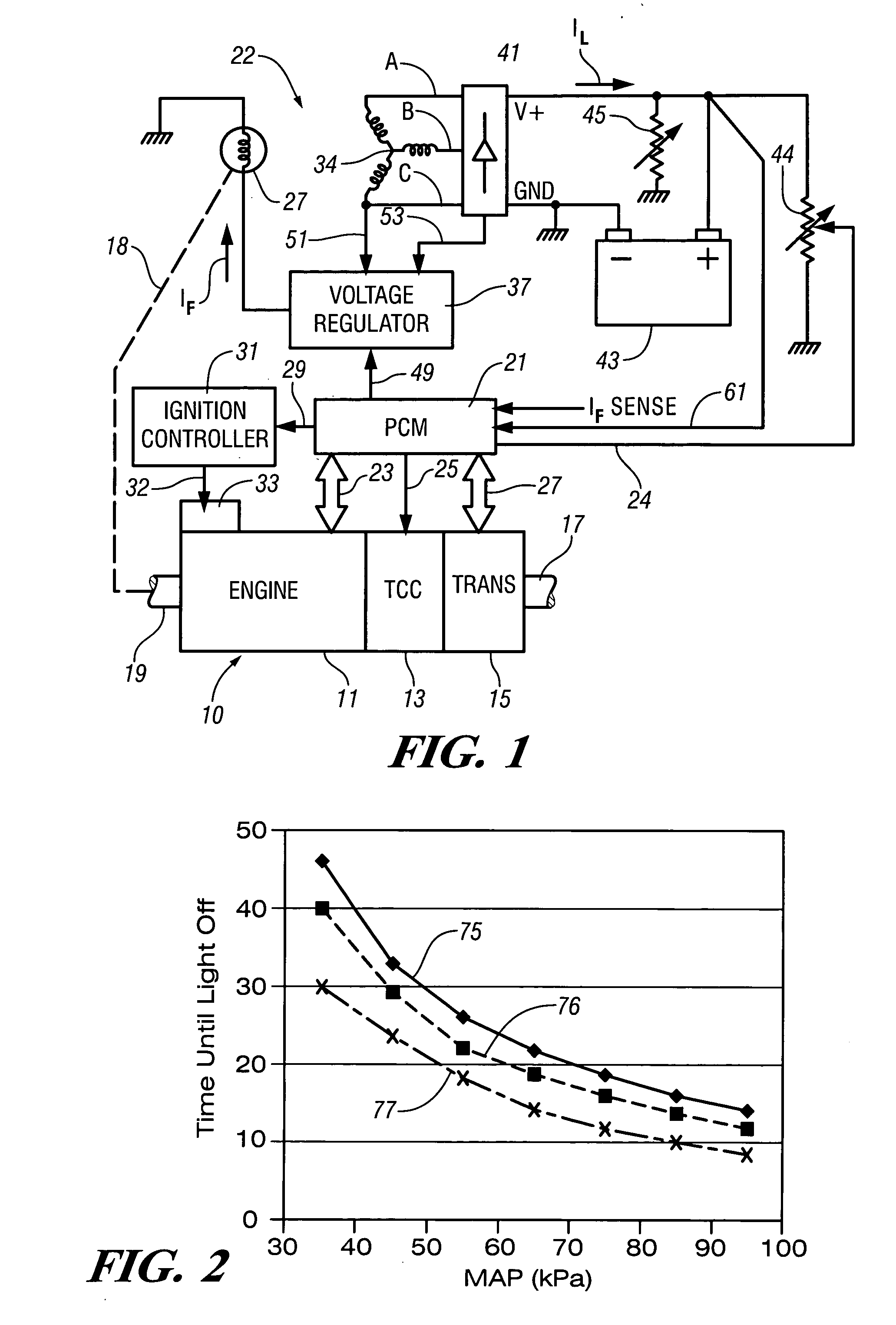
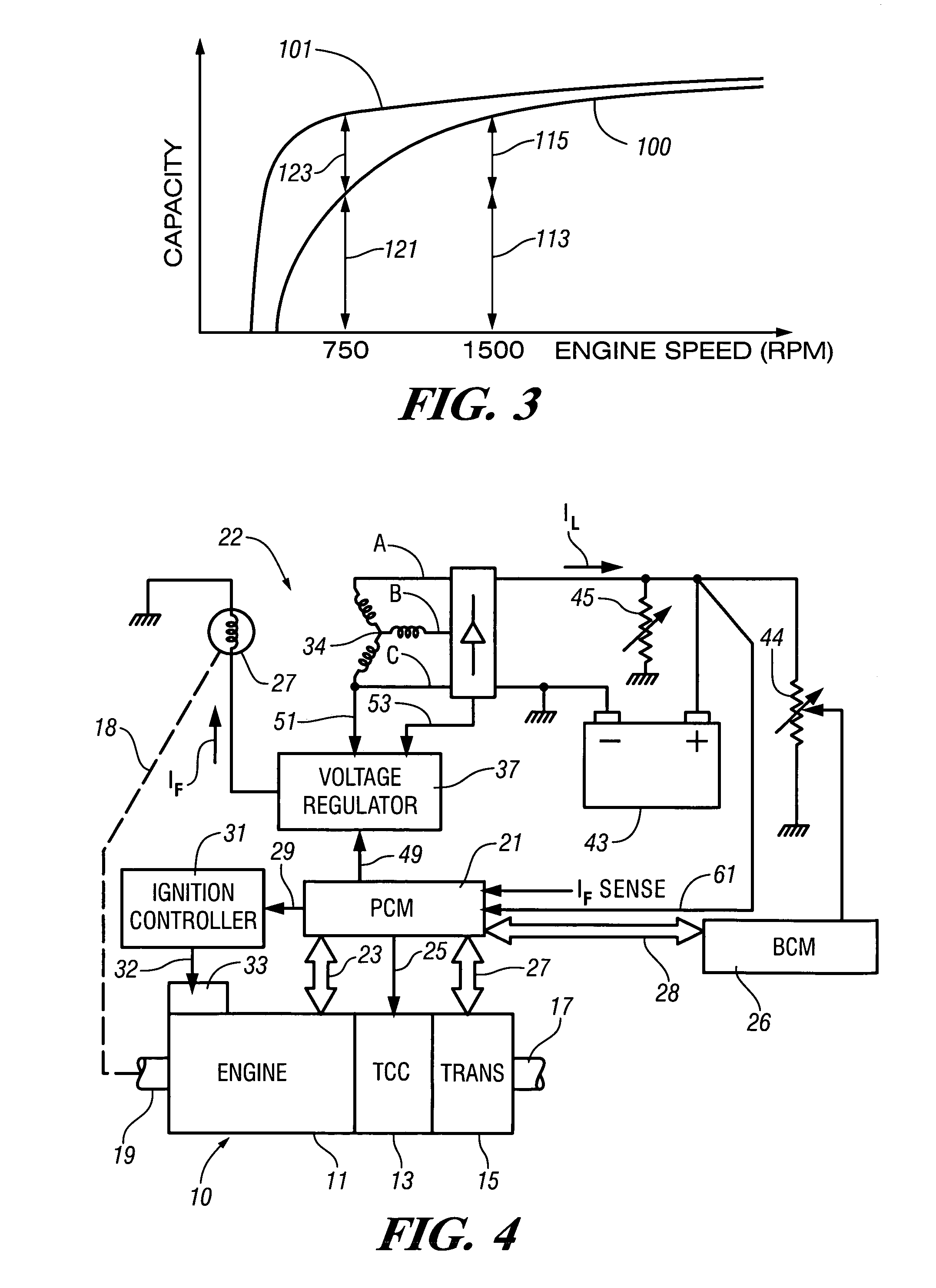
InactiveUS7007460B2Electrical controlInternal combustion piston enginesElectricityAlternative strategy
Accelerated heating of automotive exhaust system components is effected by controlling the application of supplemental electrical loads to the vehicle electrical system thereby increasing engine load and increasing exhaust heat. Alternative strategies are disclosed for implementing the invention through control of electrical and engine management system parameters.
Owner:GM GLOBAL TECH OPERATIONS LLC
Apparatus and method for accelerated exhaust system component heating
InactiveUS20050034449A1Shorten warm-up timeImprovement to cold start emissionElectrical controlInternal combustion piston enginesAlternative strategyEngine management
Accelerated heating of automotive exhaust system components is effected by controlling the application of supplemental electrical loads to the vehicle electrical system thereby increasing engine load and increasing exhaust heat. Alternative strategies are disclosed for implementing the invention through control of electrical and engine management system parameters.
Owner:GM GLOBAL TECH OPERATIONS LLC
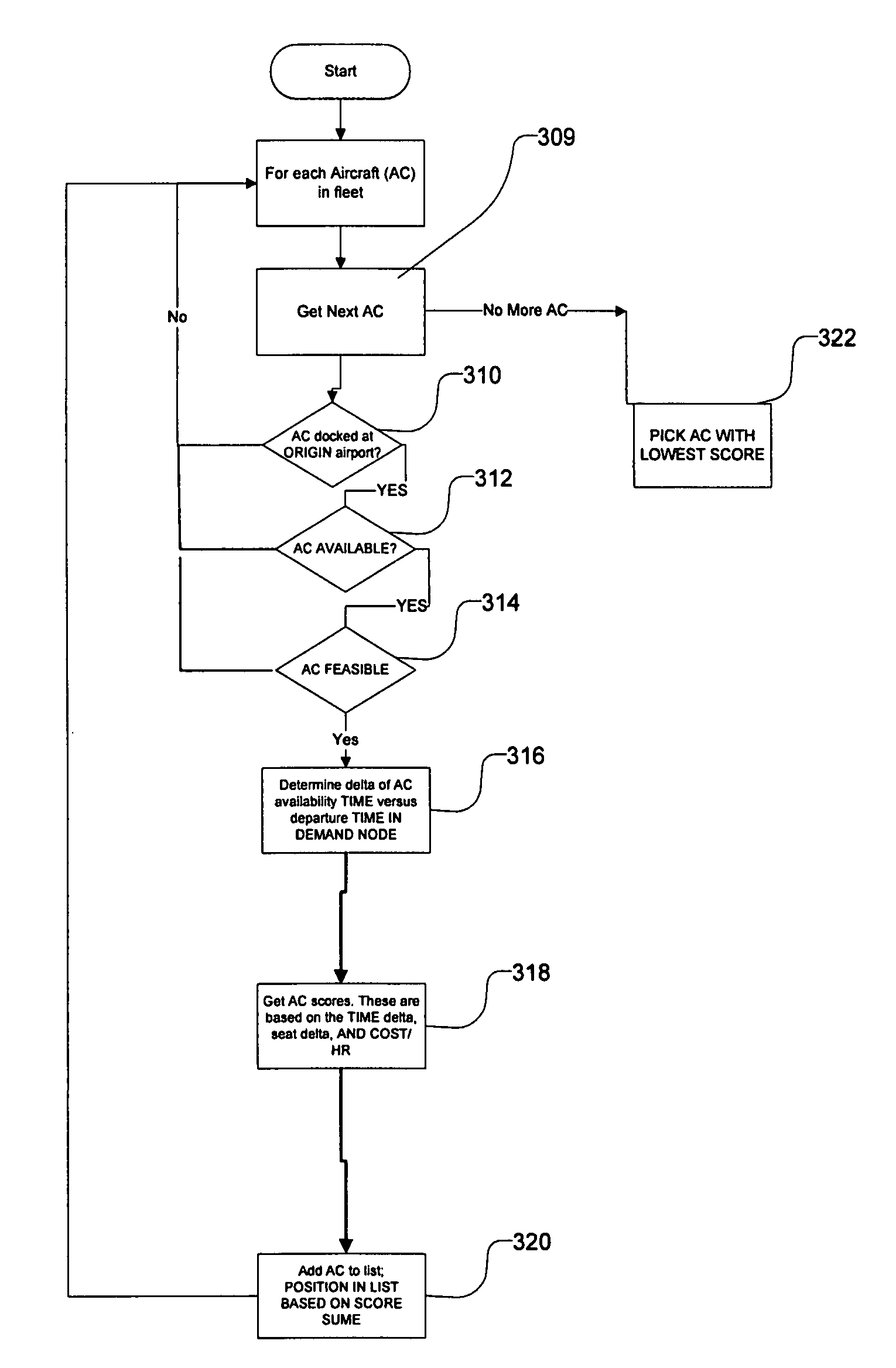
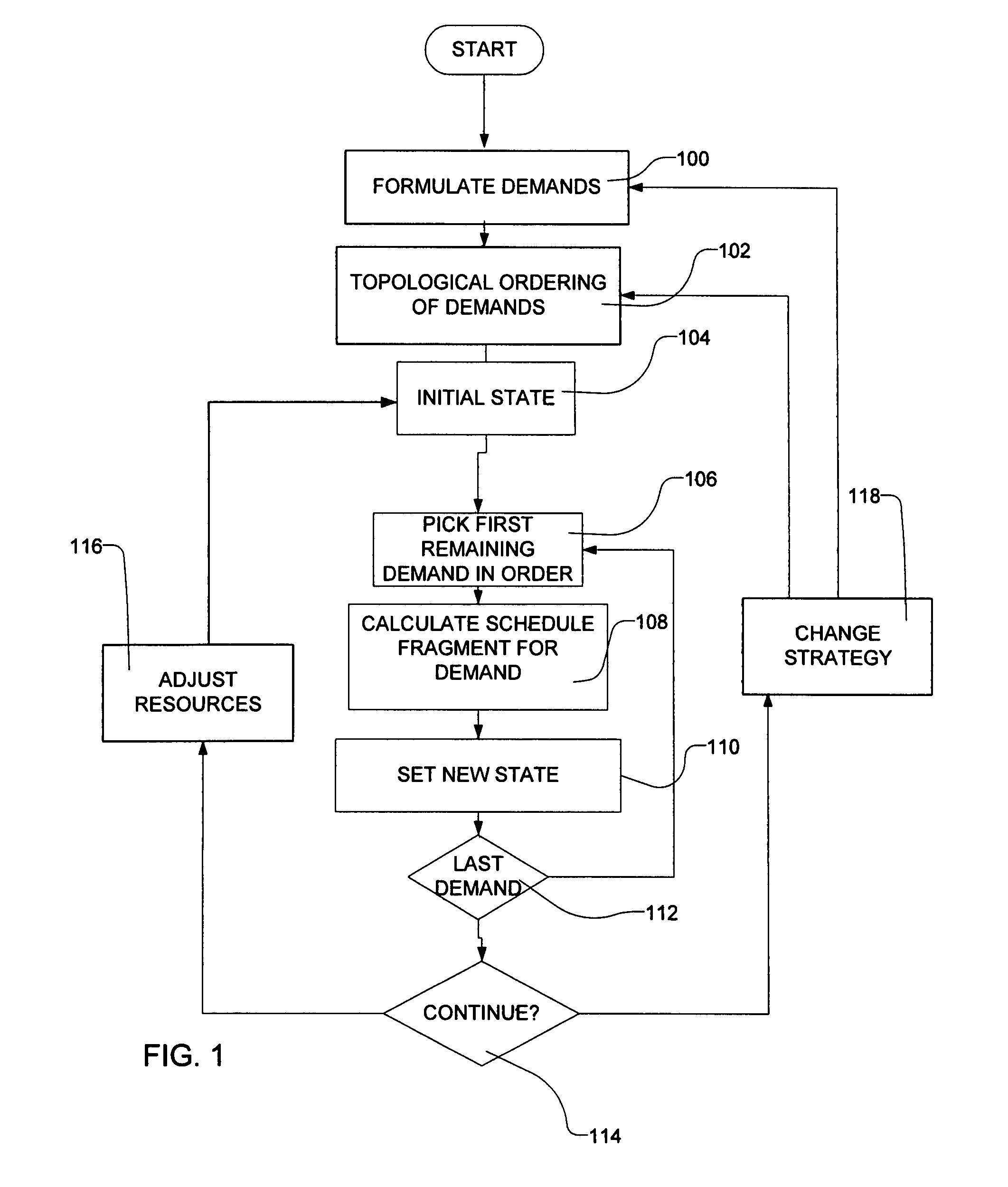
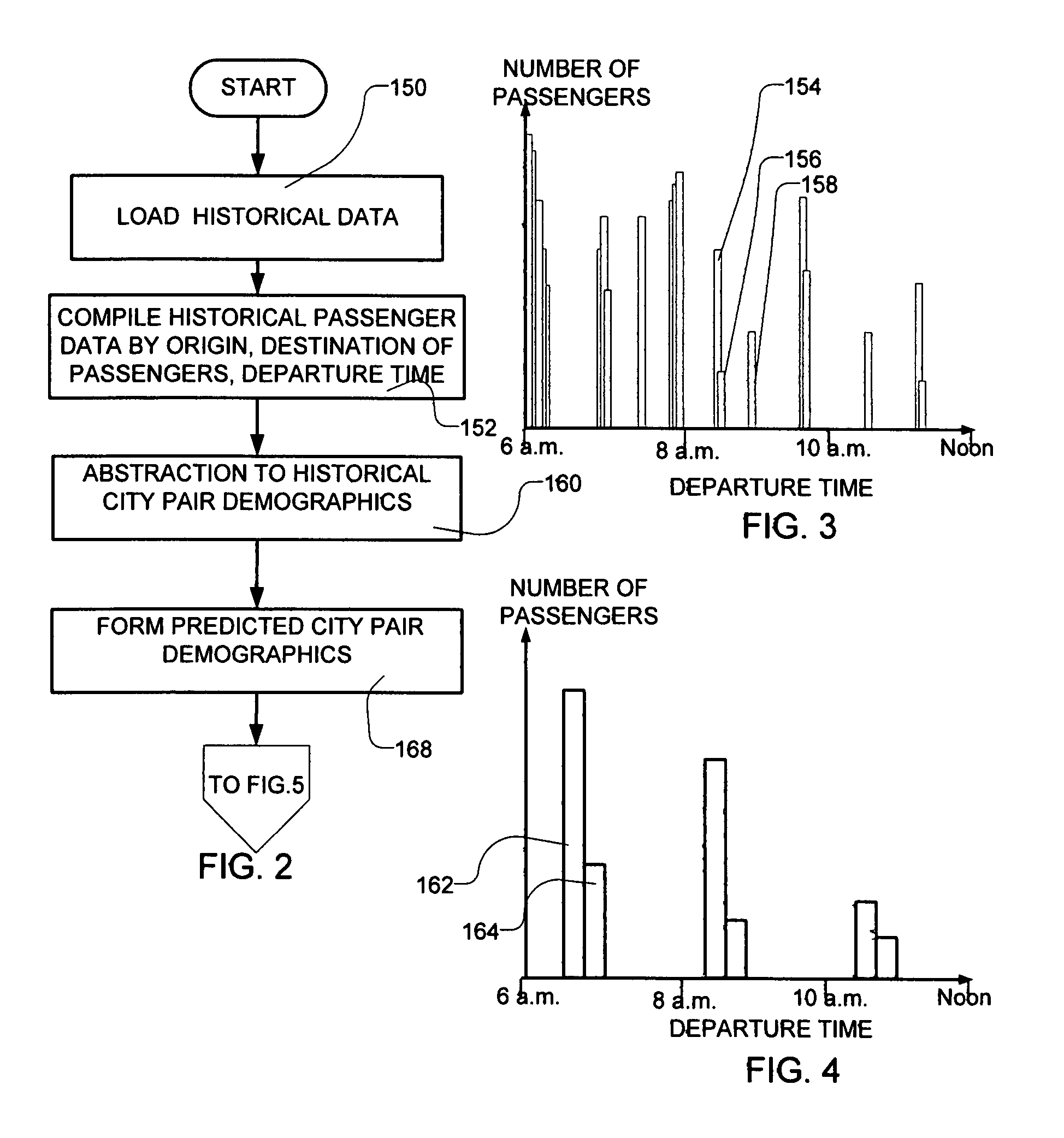
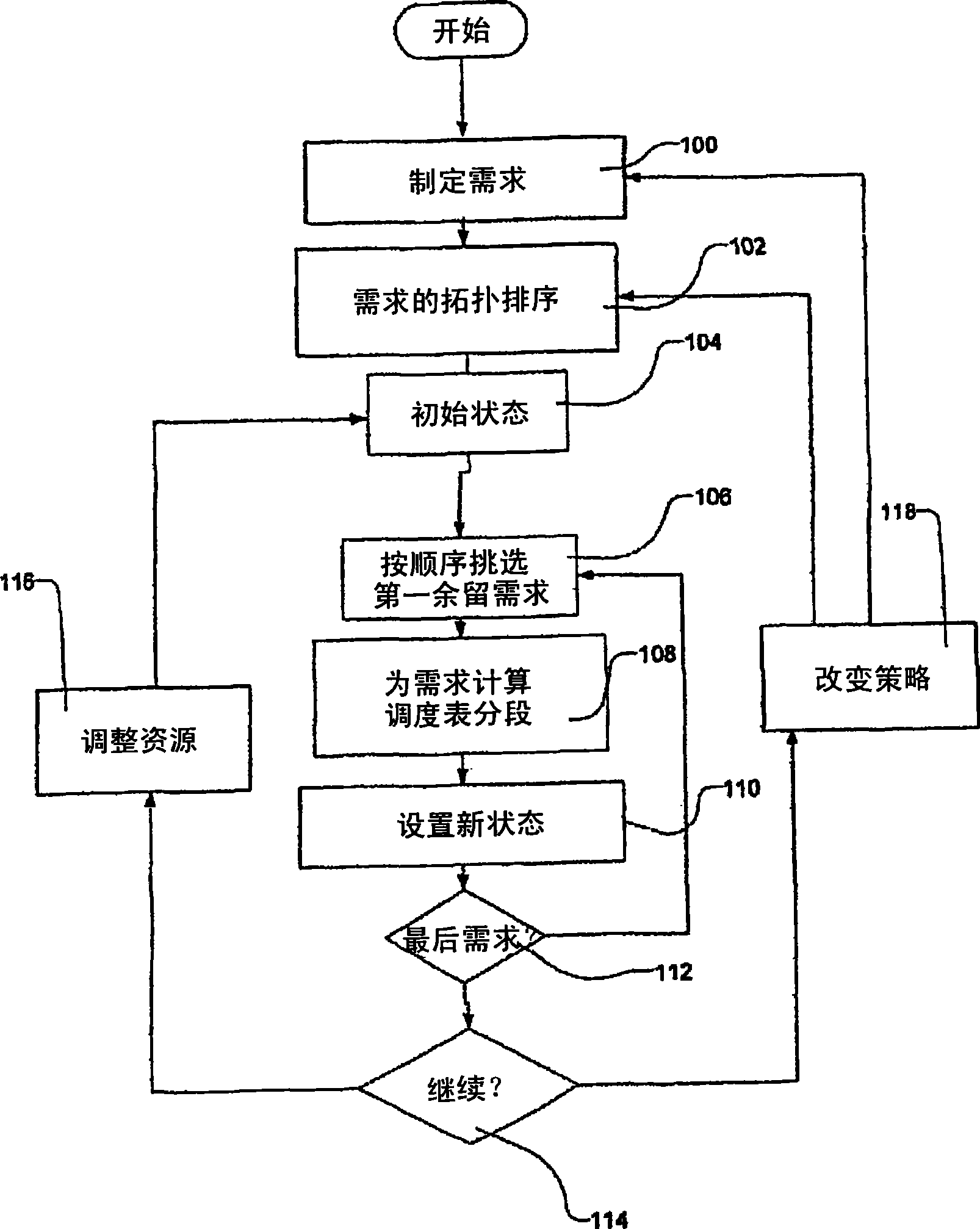
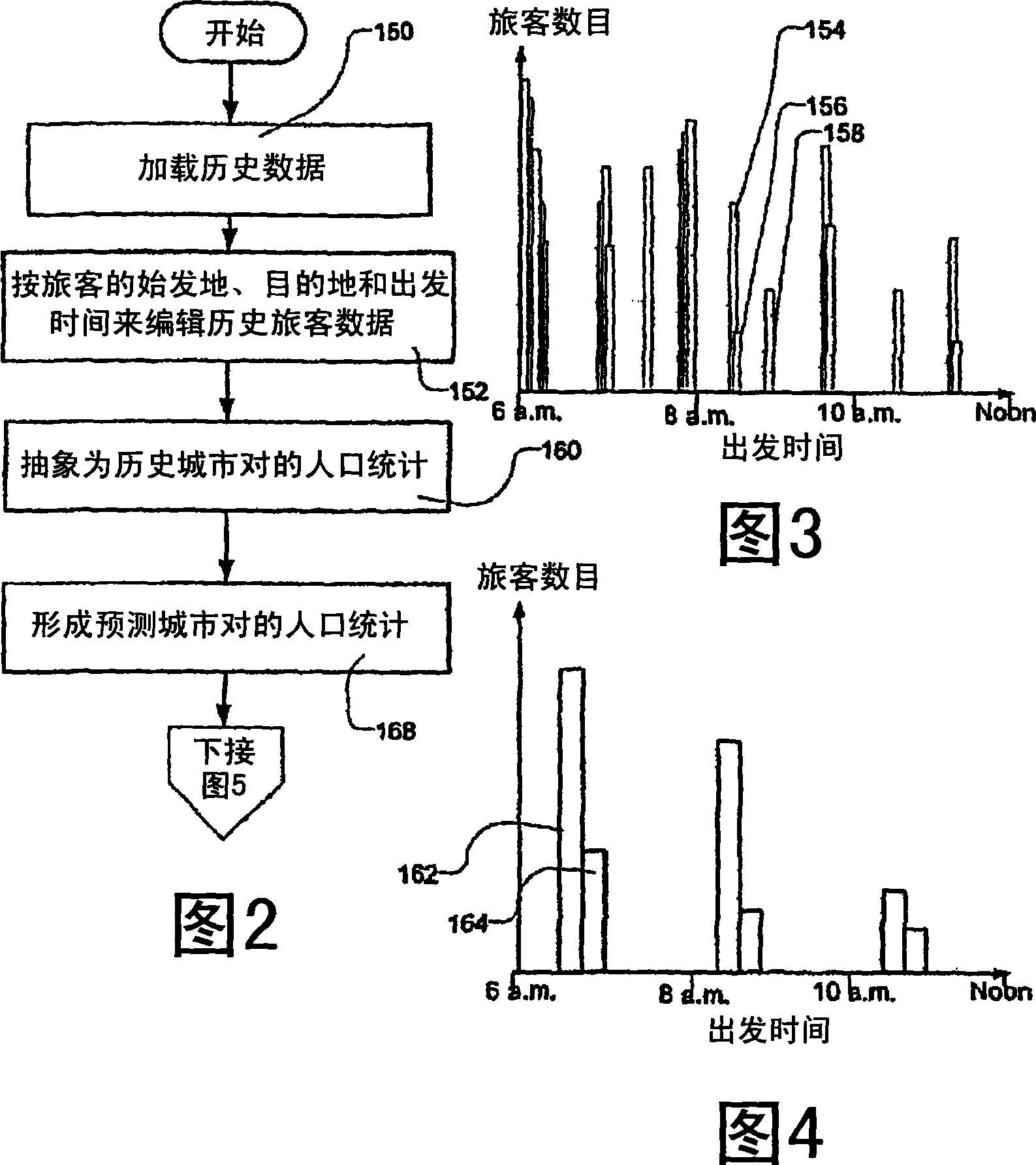
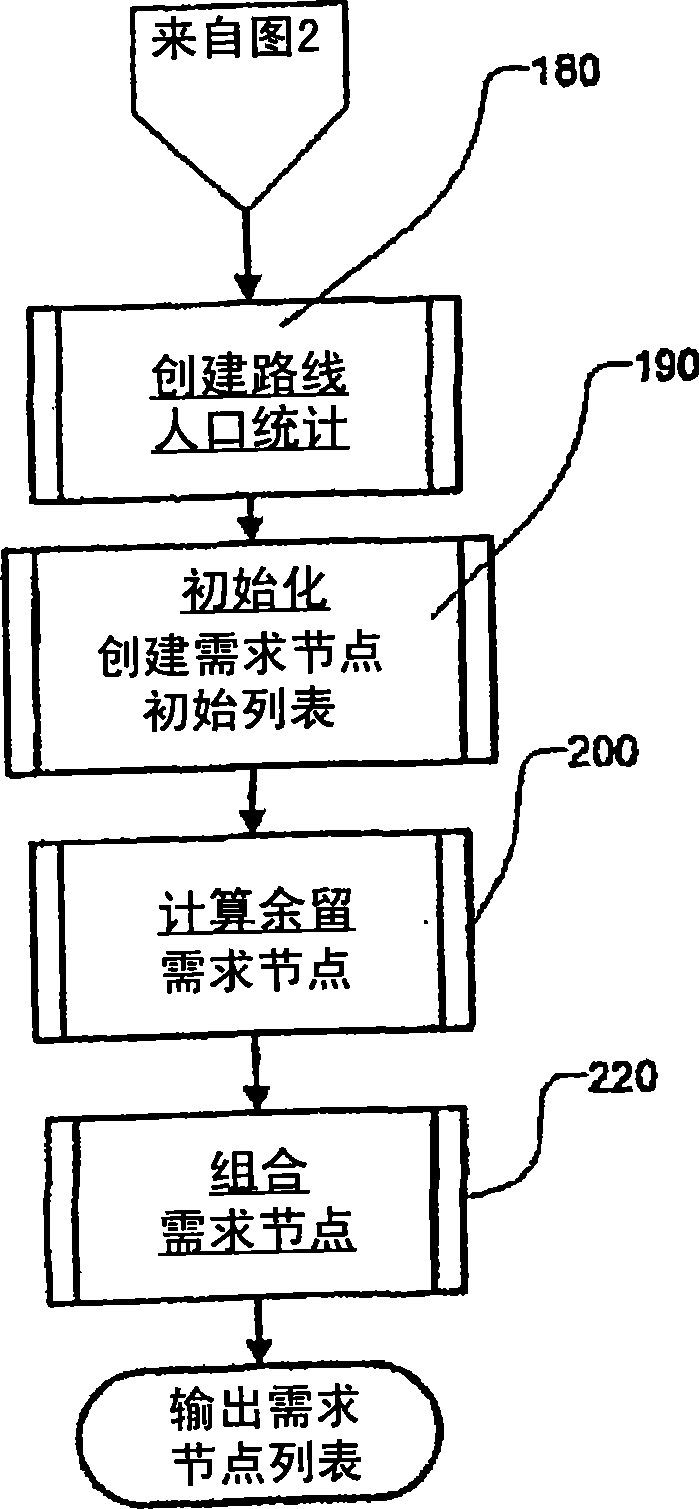
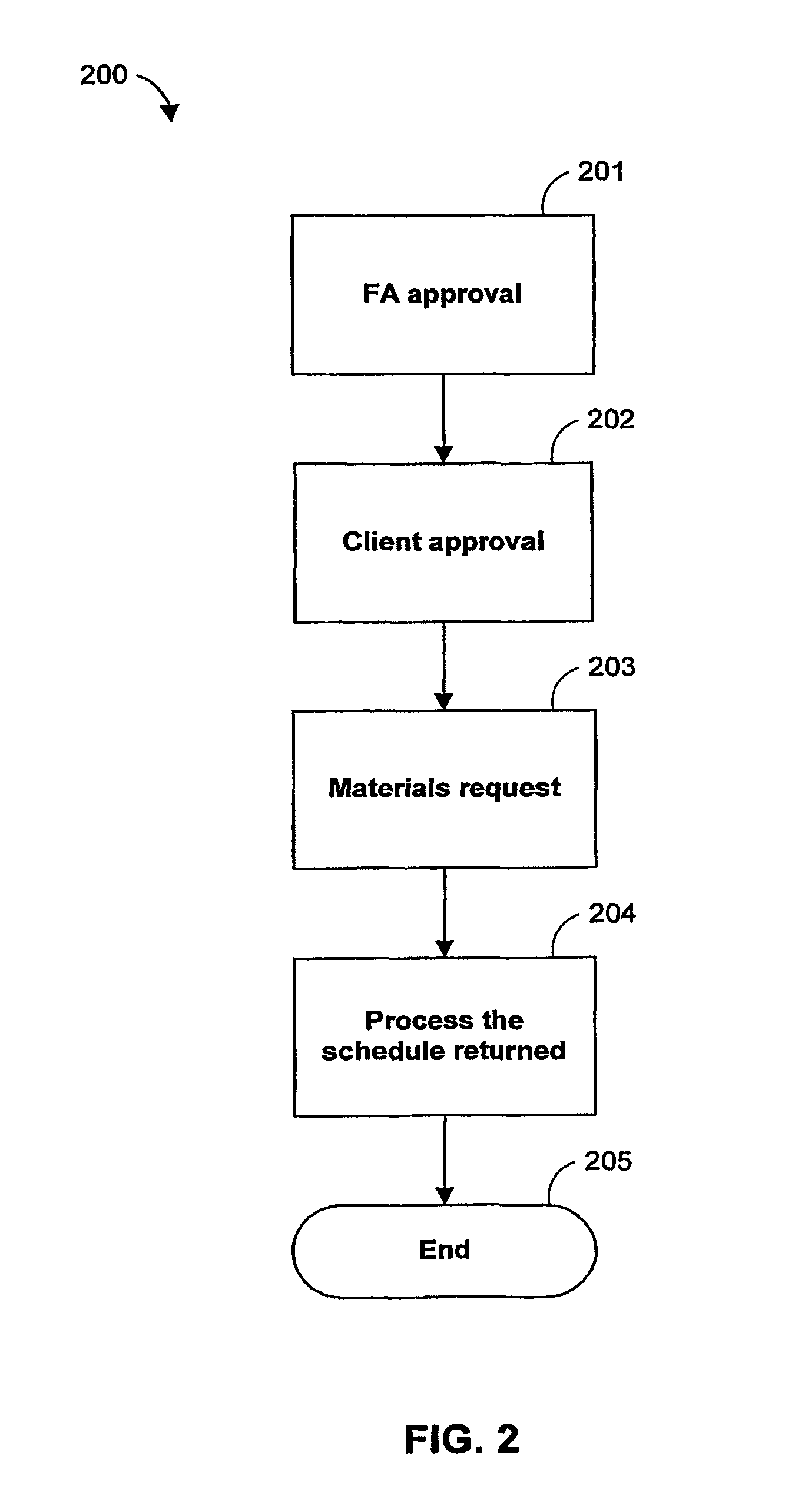
Transportation scheduling system
ActiveUS20070214033A1Realistic and usable scheduleRealistic, usable schedules rapidlyReservationsForecastingTime scheduleAlternative strategy
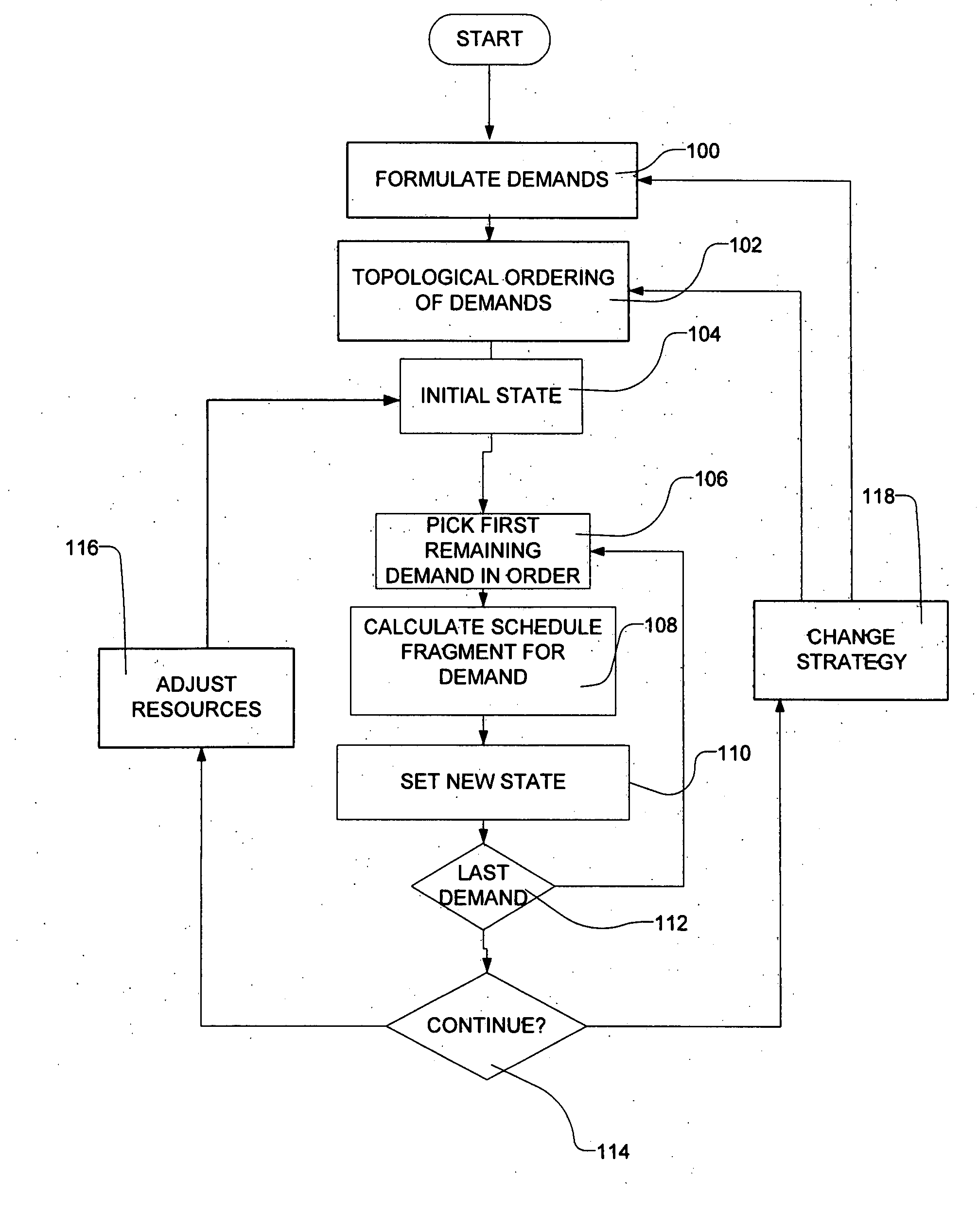
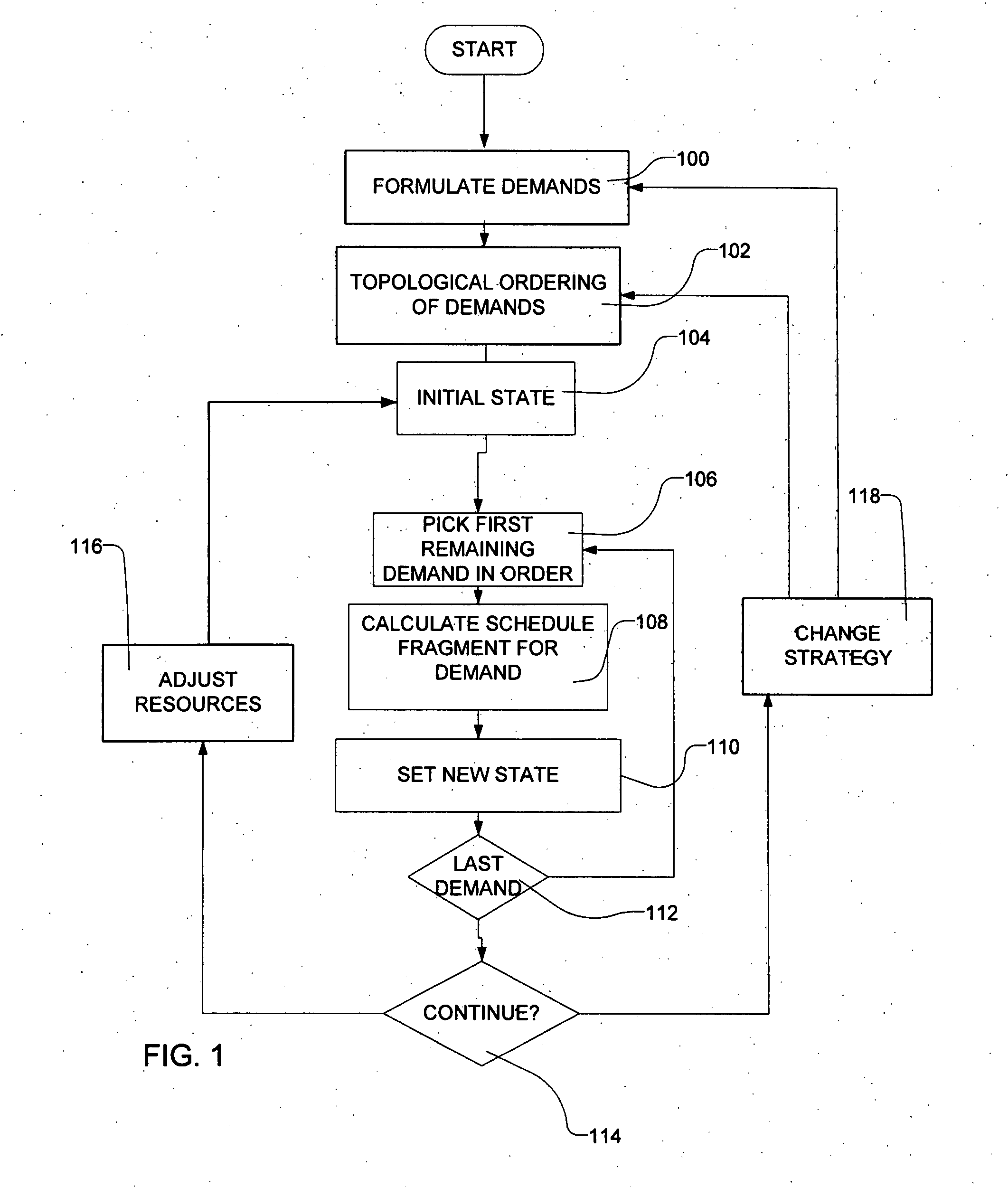
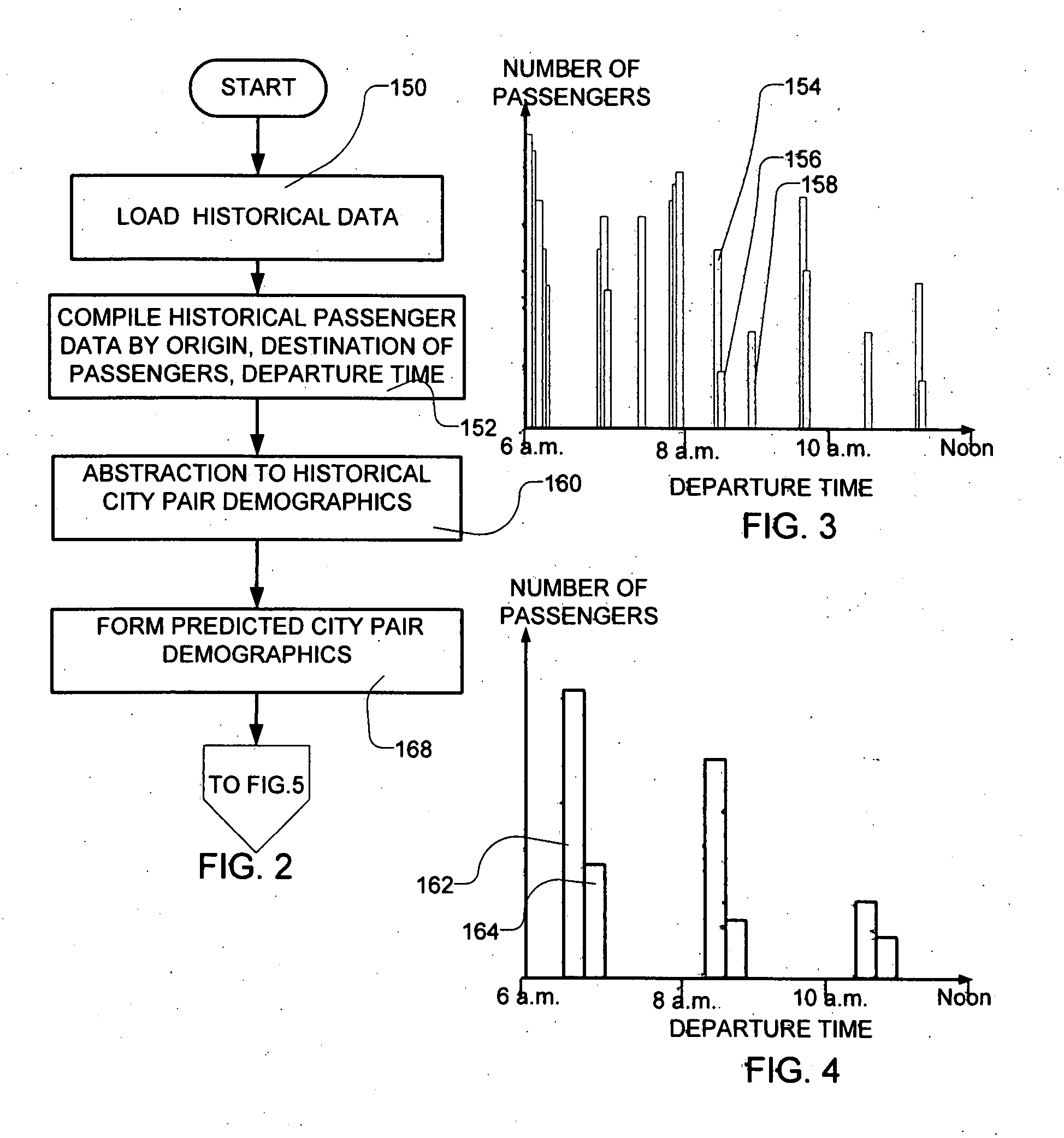
Methods for scheduling a transportation operation such as the operation of an airline. The method desirably selects a demand (100) for transportation specifying an origin, destination, and time of arrival or departure, and selects resources from a database of available resources such as aircraft (508), crew, and departure gates. The resource selection desirably is conducted so as to optimize a result function such as contribution to margin or other financial result from the particular operation specified in the demand. Upon developing a schedule fragment (108), the specified resources are committed, and the database of available resources is modified (110) to indicate that the resources are no longer available at the relevant times. The system then treats another demand and repeats the process so as to develop a full schedule. The system can develop a feasible schedule, even for a complex transportation operation in a brief time, typically in minutes or less. Schedules can be developed using alternative strategies and assumptions, and tested against one another.
Owner:INTELLIGENT IP
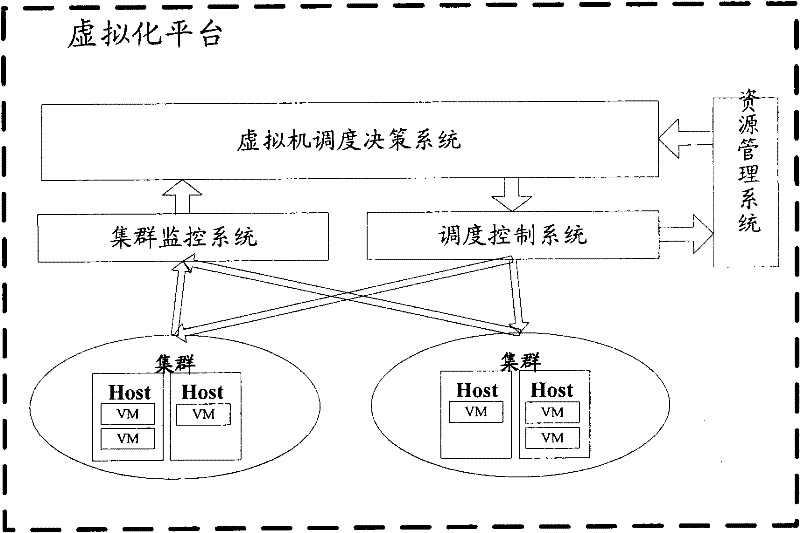
System, platform and method for virtual machine scheduling decision
InactiveCN102262567ASuit one's needsFast developmentResource allocationTransmissionOptimal decisionUser needs
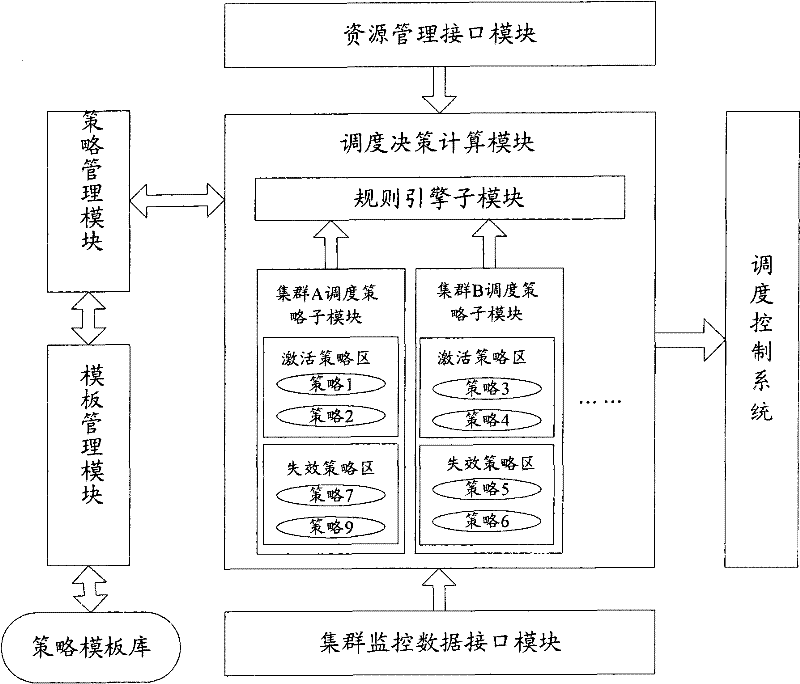
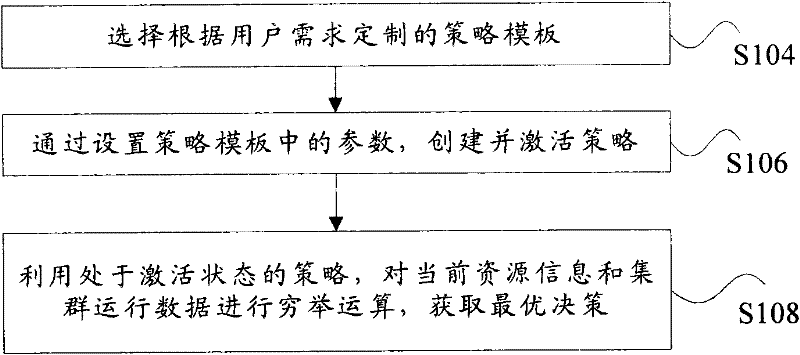
The invention discloses a system, a platform and a method for virtual machine scheduling decision-making. The above-mentioned virtual machine scheduling decision-making system includes: a template management module for selecting a policy template customized according to user needs; a policy management module for creating and activating a strategy by setting parameters in the policy template; a scheduling decision calculation module for Use the strategy in the active state to perform exhaustive calculations on the current resource information and cluster operation data to obtain the optimal decision. In the present invention, the virtual machine scheduling policy is developed and deployed using the policy template of the script, the development speed is fast and the deployment is flexible, and rich alternative policies can be provided to meet the needs of users at different levels, thereby improving the system performance of the virtual machine.
Owner:ZTE CORP
Transportation scheduling system
Methods for scheduling a transportation operation such as the operation of an airline. The method desirably selects a demand (100) for transportation specifying an origin, destination, and time of arrival or departure, and selects resources from a database of available resources such as aircraft (508), crew, and departure gates. The resource selection desirably is conducted so as to optimize a result function such as contribution to margin or other financial result from the particular operation specified in the demand. Upon developing a schedule fragment (108), the specified resources are committed, and the database of available resources is modified (110) to indicate that the resources are no longer available at the relevant times. The system then treats another demand and repeats the process so as to develop a full schedule. The system can develop a feasible schedule, even for a complex transportation operation in a brief time, typically in minutes or less. Schedules can be developed using alternative strategies and assumptions, and tested against one another.
Owner:INTELLIGENT IP
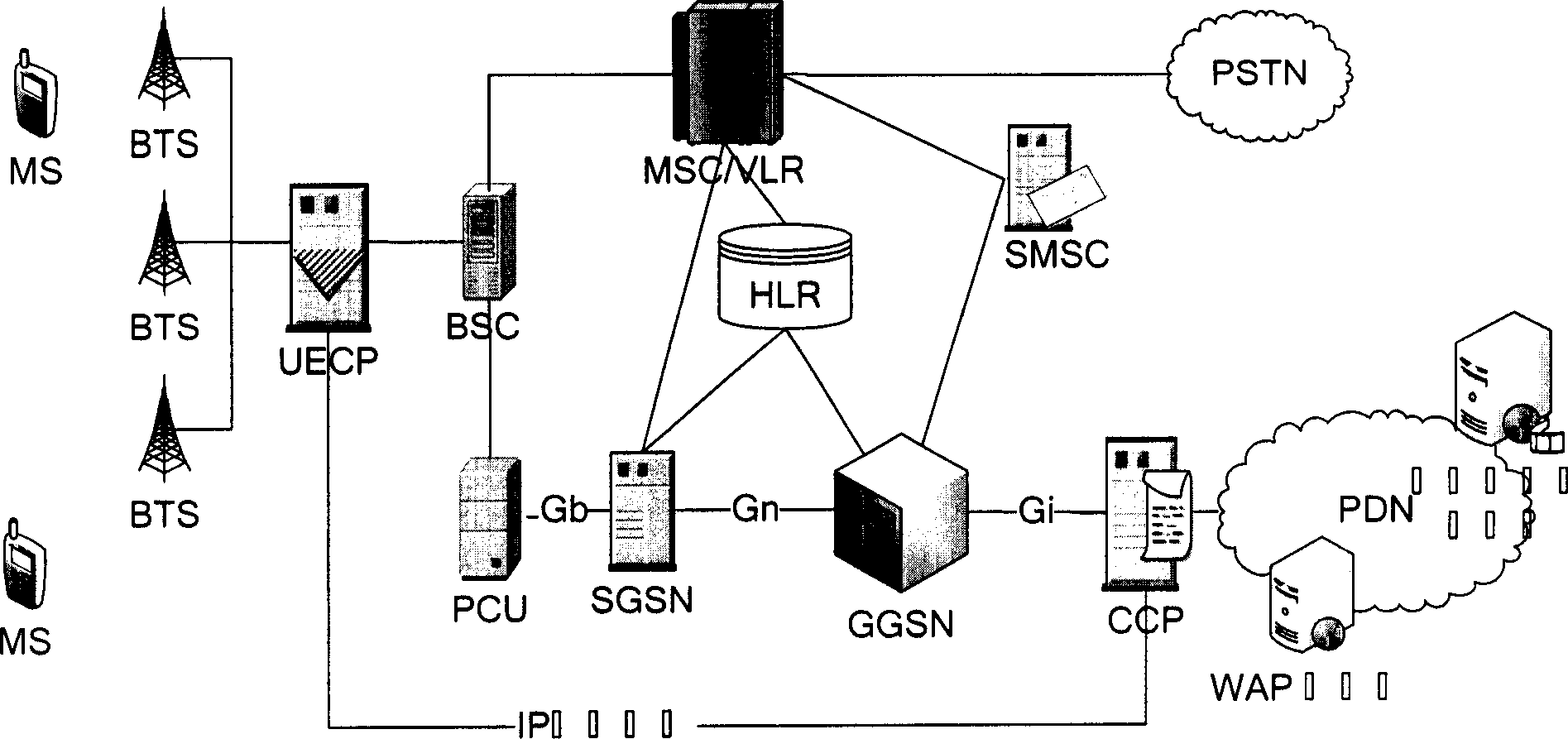
Mobile video order service system with optimized performance and realizing method
InactiveCN1791213AReduce data trafficReduce processing loadTransmissionSelective content distributionTraffic capacityAlternative strategy
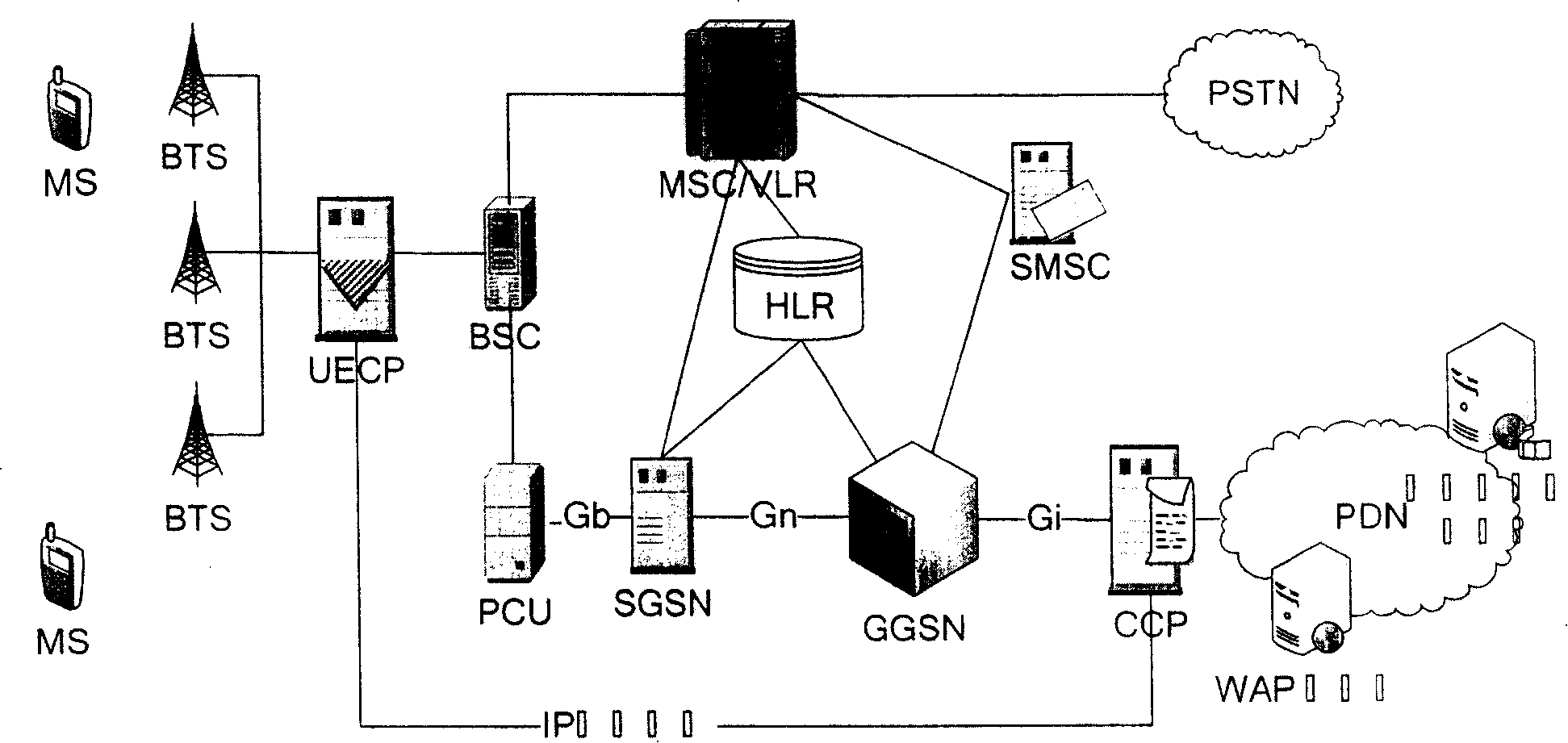
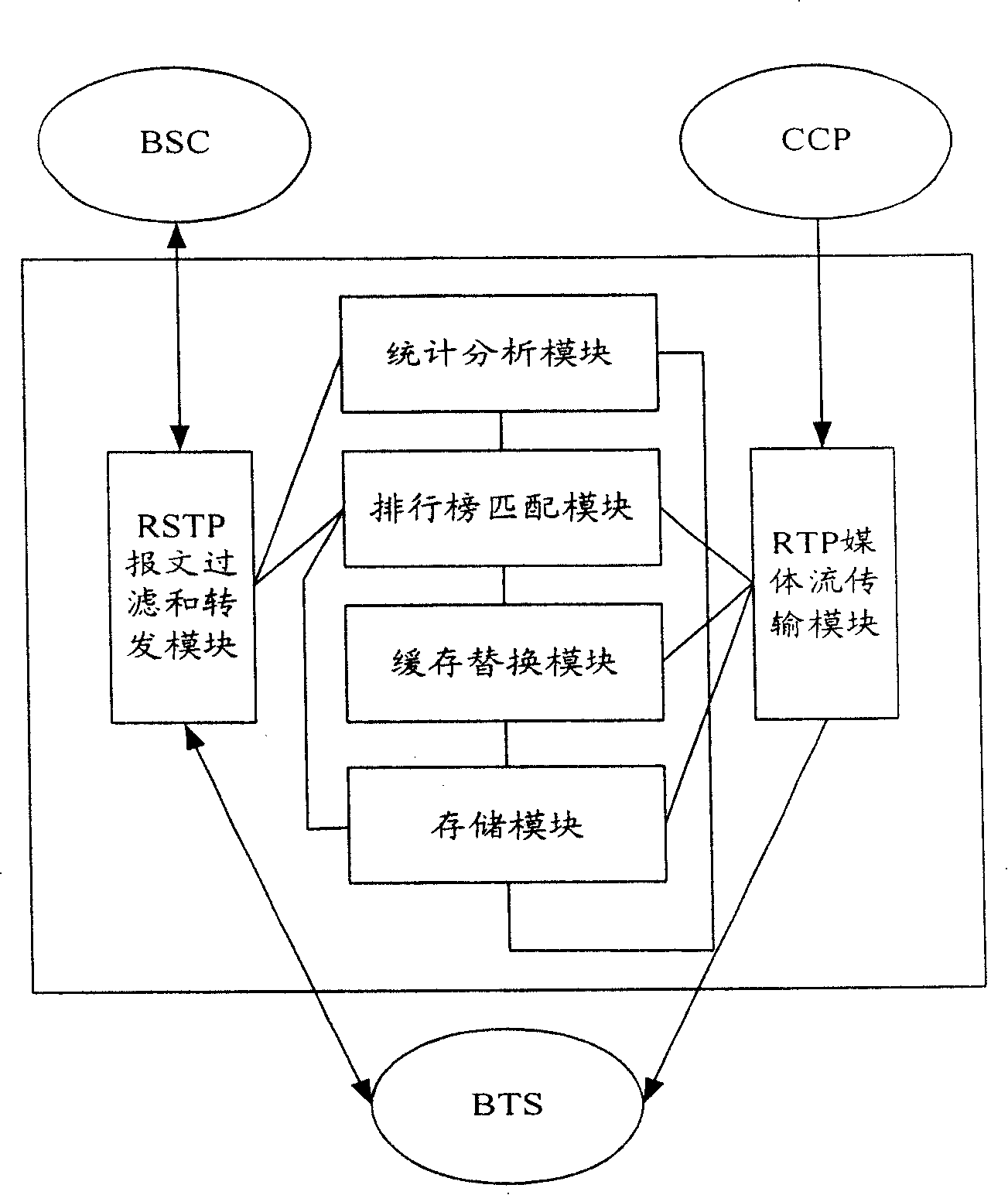
The mobile VOD business system with super performance based on GPRS mobile network comprises: besides traditional net element, a CCCP server on boundary of GGSN and PDN to release load for SCS and reduce bandwidth consumption between GGSN and SGS, a UECP server arranged in BSS that is between BSC and BTS to self-adaptive improve buffer hit rate based on buffer alternative strategy and ensure fast response to most user request, and a high-speed IP DL between CCP and UECP to transmit flow media content and construct two-stage buffer device with two proxy servers to bypass great media and reduce data flux and load for other link and eliminate the effect of VOD to network performance.
Owner:BEIJING UNIV OF POSTS & TELECOMM
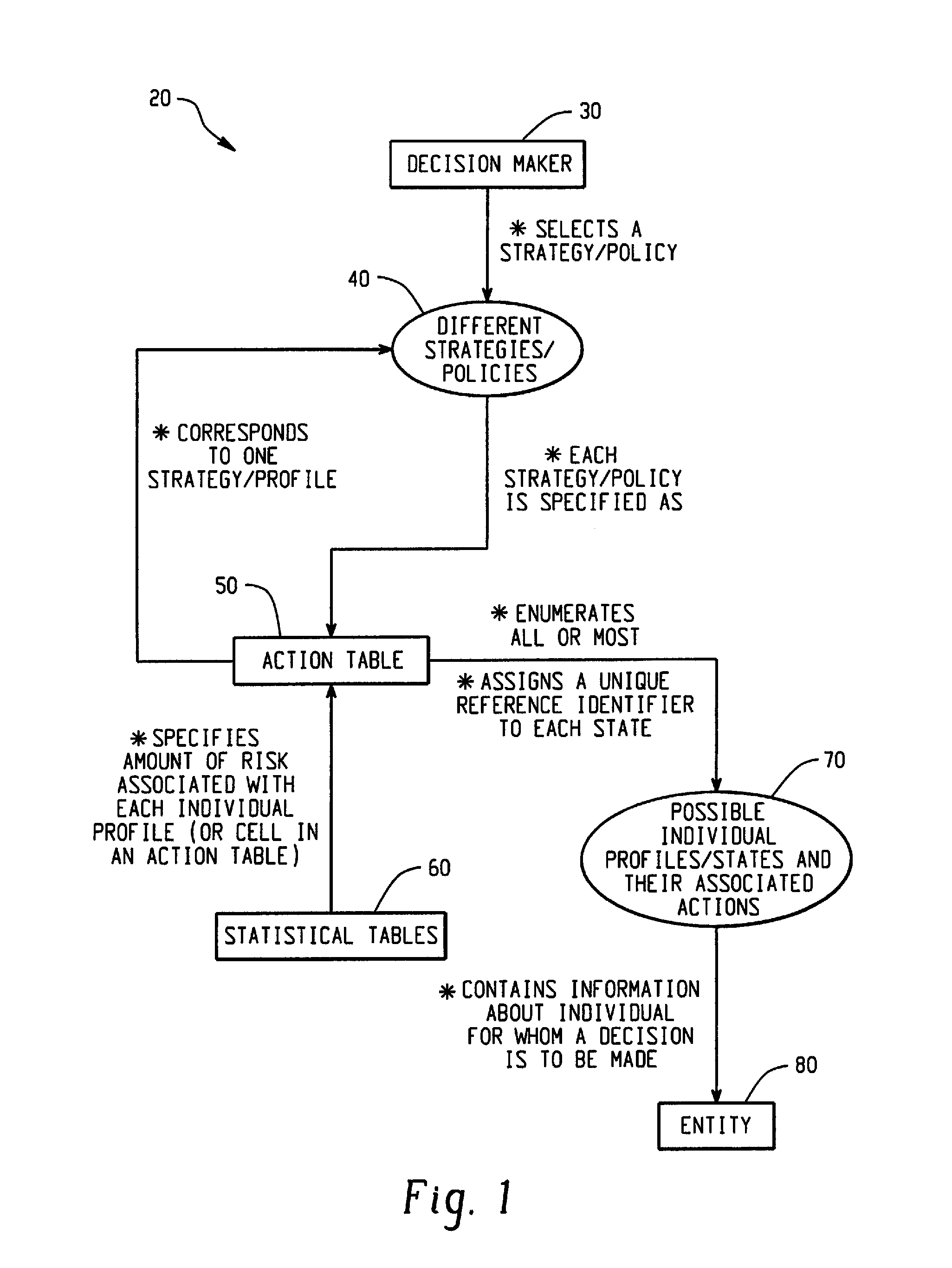
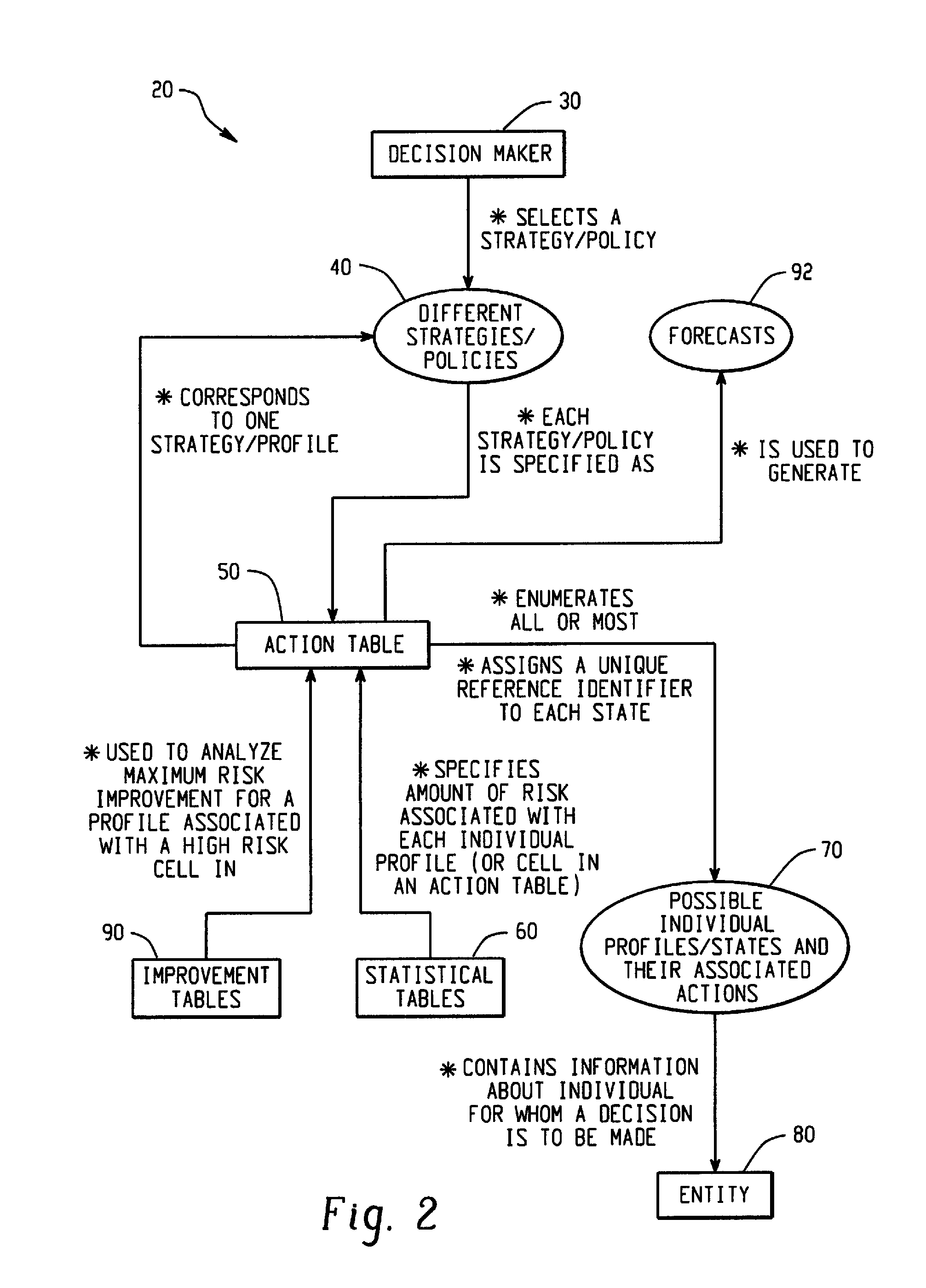
Computer-Implemented Risk Evaluation Systems And Methods
Systems and methods are provided for evaluating risks associated with alternative strategies for assessing an entity with respect to a predetermined objective. A system includes an action table that contains a plurality of possible actions that can be taken with respect to the predetermined objective for various entity profiles. One or more statistical data stores are configured to contain risk amounts associated with each entity profile, where the one or more statistical data stores are configured to contain inferred percentage distribution of applicants associated with each entity profile. One or more profile identification data stores contain entity identification information for use in determining an action for the entity. One or more improvement data stores provide an indication in the improvement in risk based upon a change in one or more characteristics for an entity profile.
Owner:SAS INSTITUTE
Transportation scheduling system
Methods for scheduling a transportation operation such as the operation of an airline. The method desirably selects a demand (100) for transportation specifying an origin, destination, and time of arrival or departure, and selects resources from a database of available resources such as aircraft (508), crew, and departure gates. The resource selection desirably is conducted so as to optimize a result function such as contribution to margin or other financial result from the particular operation specified in the demand. Upon developing a schedule fragment (108), the specified resources are committed, and the database of available resources is modified (110) to indicate that the resources are no longer available at the relevant times. The system then treats another demand and repeats the process so as to develop a full schedule. The system can develop a feasible schedule, even for a complex transportation operation in a brief time, typically in minutes or less. Schedules can be developed using alternative strategies and assumptions, and tested against one another.
Owner:DYNAMIC INTELLIGENCE INC
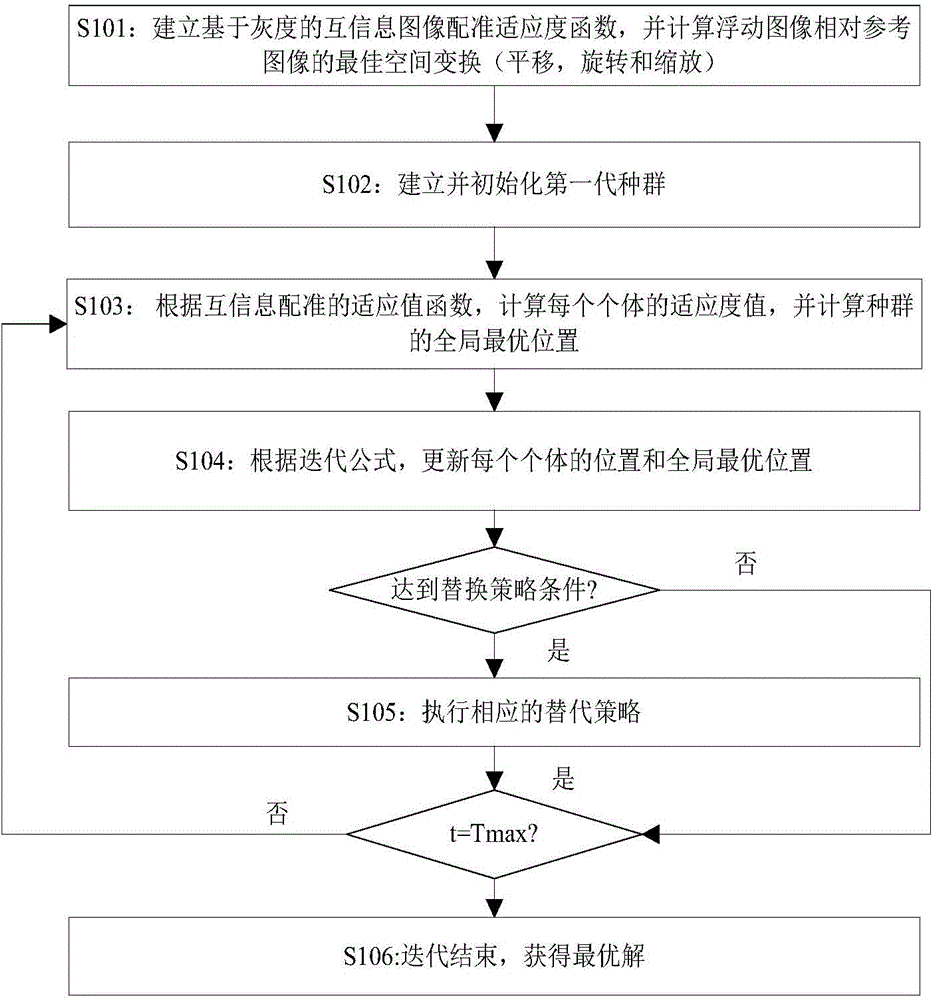
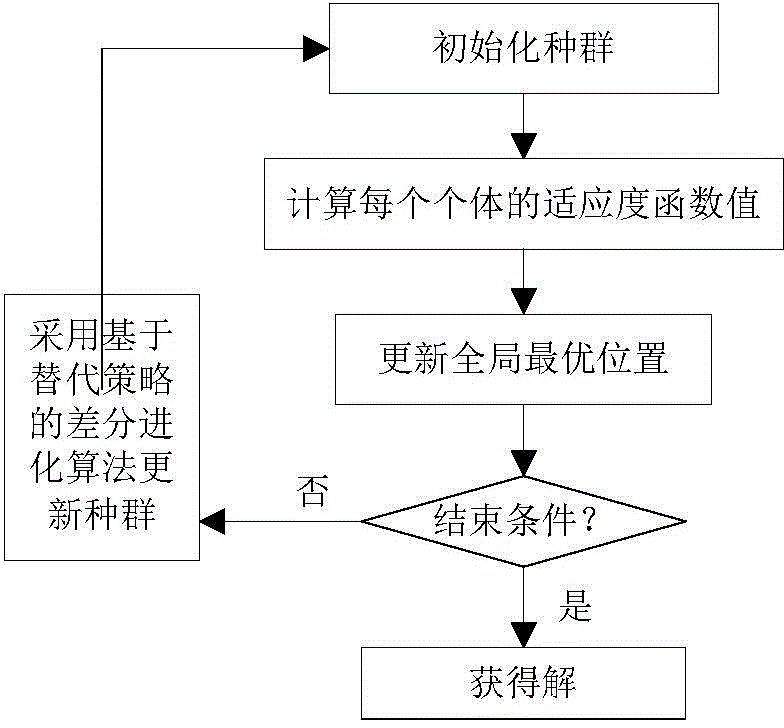
Novel image registering method
ActiveCN106204415AStrong global search capabilityImprove registration speedGeometric image transformationImaging processingAlternative strategy
The invention brings forward a novel image registering method. The method comprises the following steps: establishing gray-scale-based mutual information registering adaptation value function; establishing and initializing a group, wherein four dimensions of each individual in the group respectively represents horizontal translation, vertical translation, a rotation angle and a zoom coefficient of a floating image; according to the mutual information registering adaptation value function, calculating a fitness value of each individual, and calculating an optimal position of the whole group; by use of an iteration mechanism of a differential evolution algorithm, updating a position vector of each individual, and updating the optimal position of the whole group; determining whether conditions for executing an alternative strategy is satisfied, and if so, executing the corresponding alternative strategy; and repeatedly executing the aforementioned steps until maximum iteration frequency Tmax of the differential evolution algorithm is satisfied. The novel image registering method has the advantages of good registering stability and high precision, greatly improves the performance of an image registering algorithm and lays a reliable foundation for subsequent image processing work.
Owner:NANJING UNIV OF POSTS & TELECOMM
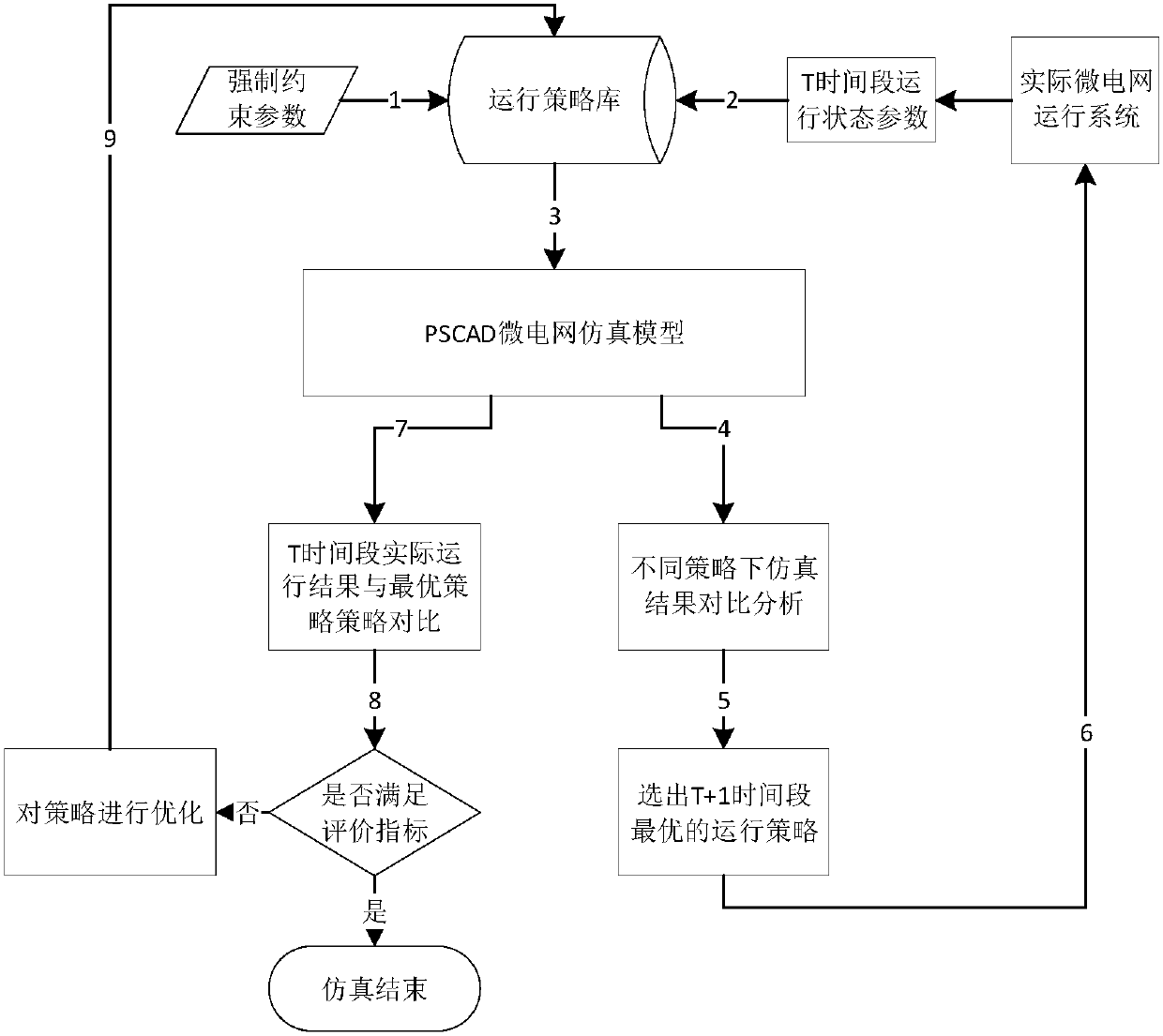
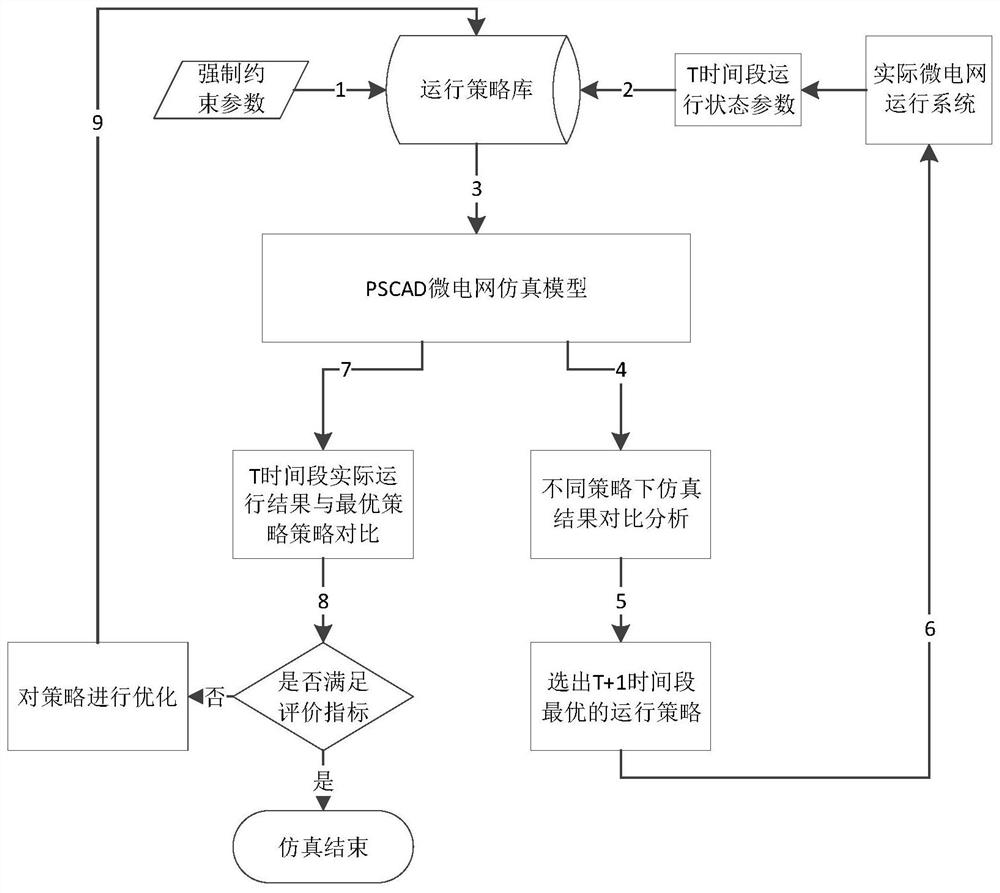
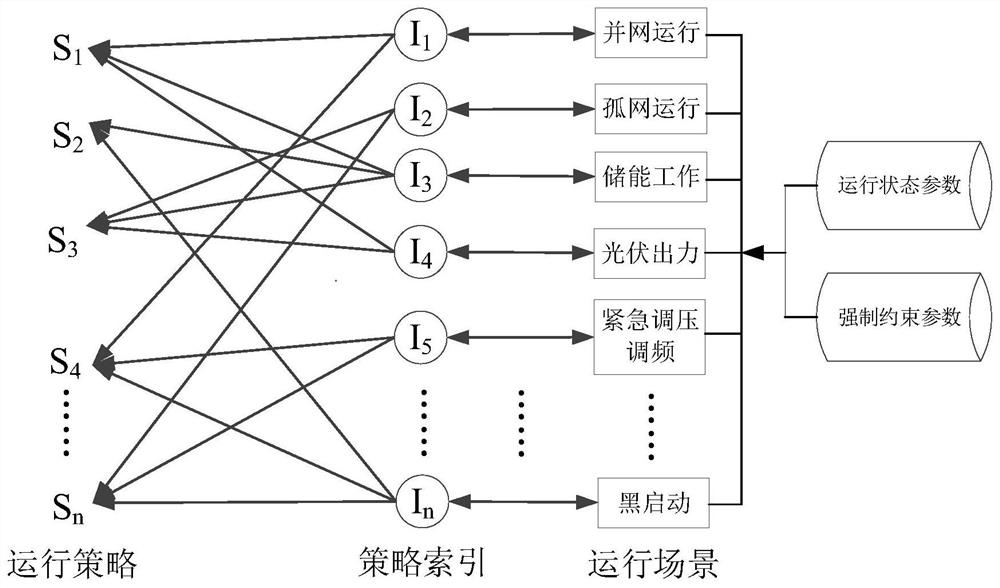
Microgrid-based dynamic strategy simulation and optimization method
ActiveCN108288855AImprove adaptabilityImprove reliabilitySingle network parallel feeding arrangementsAc network load balancingMicrogridAlternative strategy
The invention discloses a microgrid-based dynamic strategy simulation and optimization method. The microgrid-based dynamic strategy simulation and optimization method comprises the steps of building amicrogrid running strategy library to store and manage control strategies of a microgrid in different running environments; dividing control time frames during the running process of a microgrid system, and timely selecting an alternative strategy set for running at a next time frame from the strategy library according to states of each power supply and a load in the microgrid; issuing the selected strategy set to a microgrid simulation model built on the basis of MATLAB, performing simulation calculation on the running state of the microgrid, and selecting the optimal running strategy as therunning strategy of the next time frame by comparing and analyzing a result of the simulation system under each alternative strategy; and comparing and analyzing the strategy running result and the simulation calculation result, improving and optimizing the employed strategy by a genetic algorithm, and favorably achieving the optimal effect during application after iteration.
Owner:GUODIAN NANJING AUTOMATION
Determination of chemical or pysical properties of sample or component of a sample
ActiveUS20090024360A1Reduced mechanical stabilityShorten speedRadiation pyrometryInterferometric spectrometrySpectroscopyCompound (substance)
The present invention offers an alternative strategy for the correlation of interference information to chemical and / or physical properties of a sample. This strategy can be implemented in a method and a system, which offer substantial technical and commercial advantages over state of the art techniques based on interference spectroscopy. The method comprises the steps of: a. obtaining an interferogram and / or at least one interferogram element corresponding to a modulation of electromagnetic signal emitted from, transmitted onto or through, or having interacted with at least a part of the sample, b. performing i. at least one transformation of the interferogram and / or a segment of the interferogram and / or an interferogram element with at least one function, ii. optionally repeating i) for another segment of the interferogram and / or interferogram element, wherein the transformation does not comprise a Fourier Transformation if i) is conducted only once, thereby obtaining at least one score, c. correlating said at least one score to the at least one chemical or physical property.
Owner:CHEMOMETEC AS
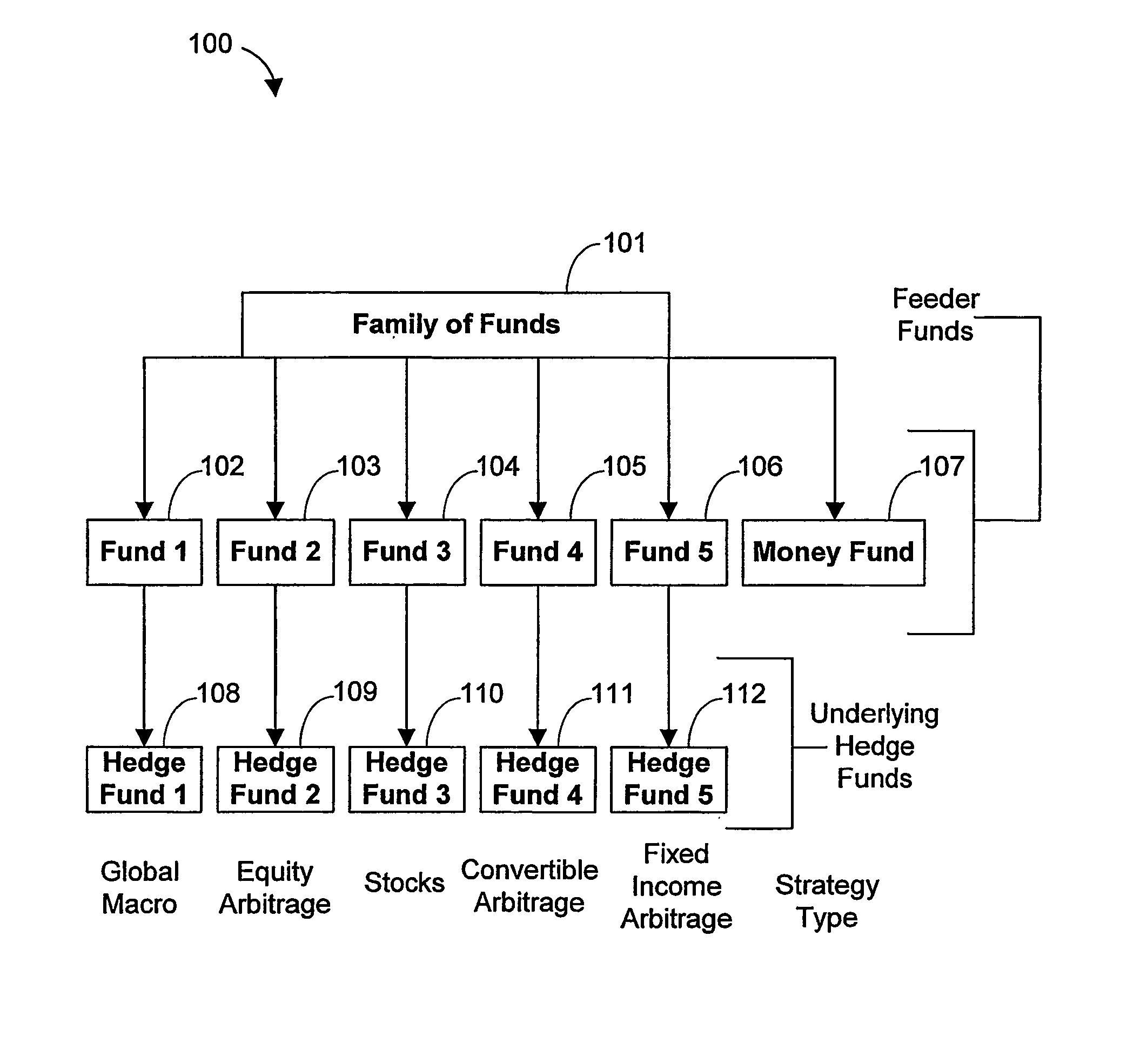
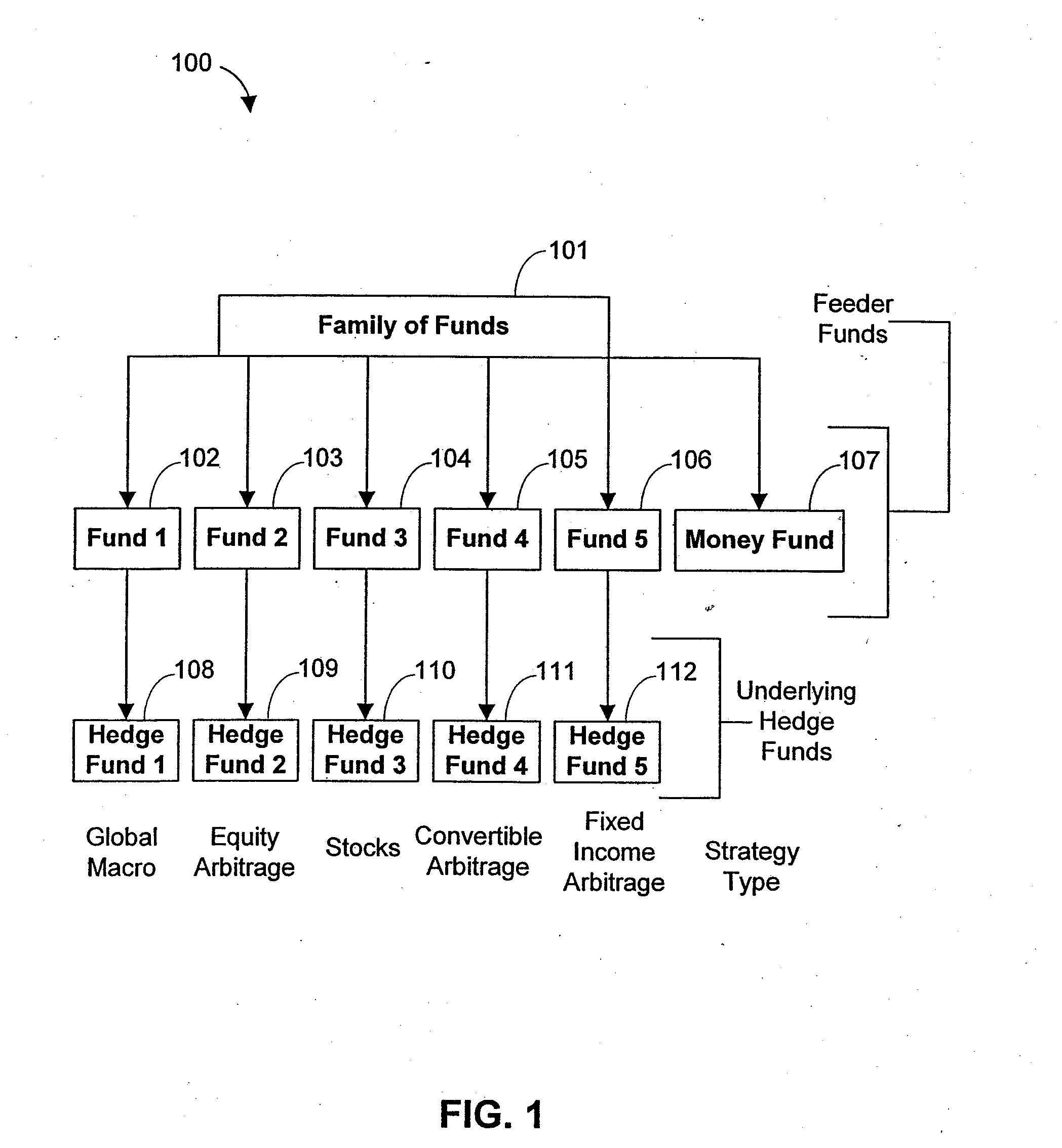
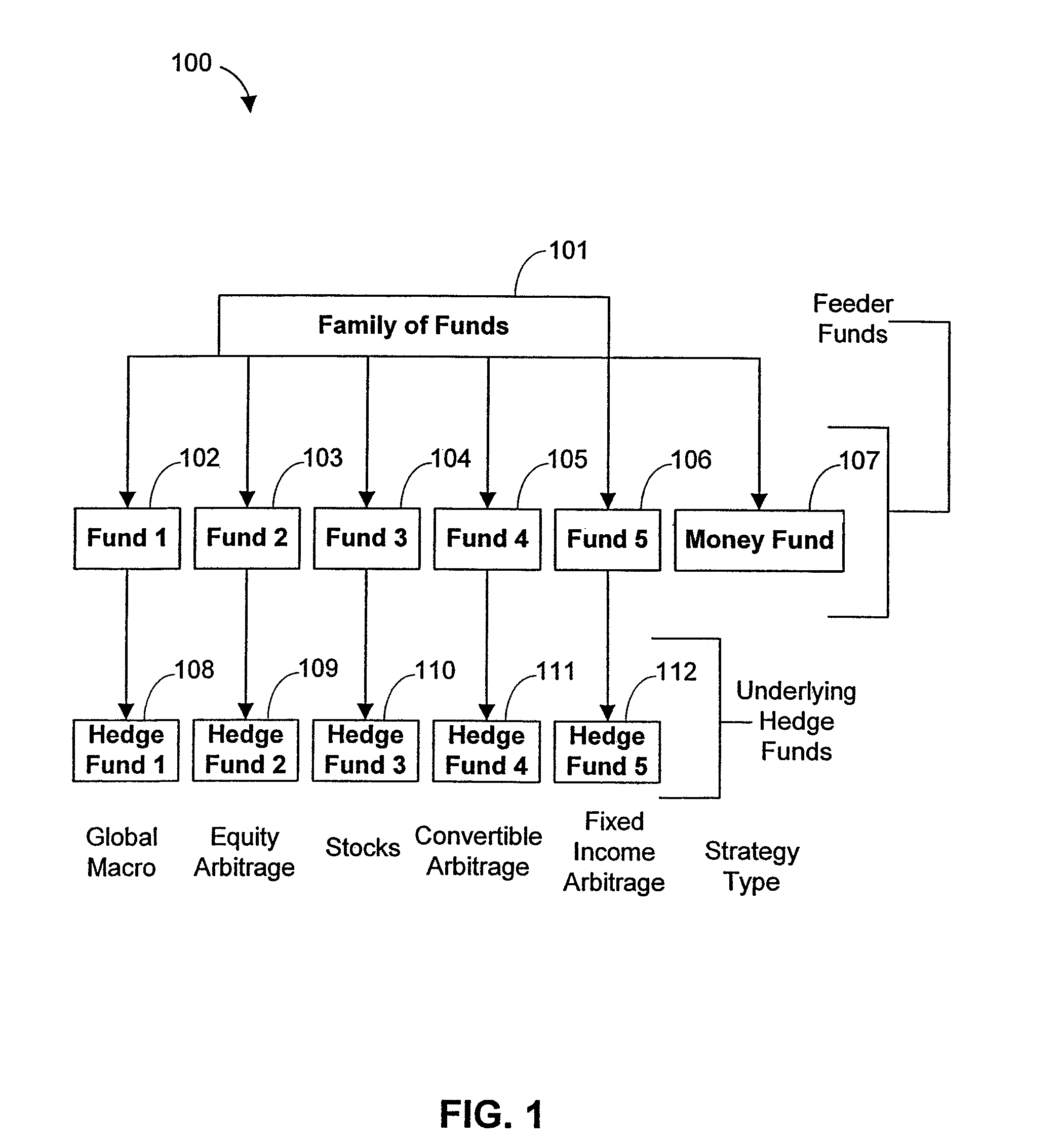
Systems and methods for offering and servicing hedge funds
ActiveUS8024246B2Improve liquiditySimplified documentationFinanceCommerceAlternative strategyData science
A family of hedge funds, serving as “feeder funds” into underlying single-manager hedge funds, formed to provide smaller investors with the ability to allocate and reallocate assets among alternative strategies, and this basic structure combining interrelated systems and methods for offering, redeeming, exchanging, valuing, reporting and servicing the same is a new approach. The system and methods described herein provide investors having less than ultra-high net worth portfolios with access to hedge funds and the potential valuable diversification to an overall portfolio, and the ability to customize their portfolio of hedge funds to their individual needs and adjust such portfolio over time as seen fit in light of changing financial needs and market conditions. This invention gives a wide range of investors access to hedge funds, creating economic value using a new source of stable investor capital for hedge fund managers, a value shared with investors through reduced costs.
Owner:BANK OF AMERICA CORP
Application of exosome secreted by human umbilical cord mesenchymal stem cell in preparing medicine for repairing skin ultraviolet injury
InactiveCN108392494AInhibit apoptosisEasy to synthesizeMammal material medical ingredientsDermatological disorderDiseaseAlternative strategy
The invention belongs to the technical field of skin light damage repair, and discloses application of exosome secreted by a human umbilical cord mesenchymal stem cell to preparation of a medicine forrepairing skin ultraviolet injury. The umbilical cord mesenchymal stem cell exosome is a natural vesica, so that the immunogenicity is lower. Meanwhile, the exosome has functions of oxidation resistance, antiinflammatory effect, proliferation, apoptosis inhibition, collagen synthesis and the like similar to the functions of a parent cell in tissue regeneration repair, and can play a role in skinprotection in multiple ways. Therefore, the exosome hopefully become an alternative strategy for decellularization treatment, and has important clinic application prospect and market transformation value in multiple skin regeneration related diseases.
Owner:JIANGSU UNIV
Methods for treating tumors and cancerous tissues
InactiveUS20110217270A1Improve bioavailabilityBiocideGenetic material ingredientsApoptosisAlternative strategy
The invention disclosed herein relates generally to immunotherapy and, more specifically, to therapeutic methods for treating tumors and cancerous tissues by first inducing necrosis or apoptosis (e.g., cryotherapy, chemotherapy, radiation therapy, ultrasound therapy, or a combination thereof applied against at least a portion of the tumor or cancerous tissue), and then delivering one or more se doses of antigen presenting cells (e.g., autologous dendritic cells) intratumorally or proximate to the tumor or cancerous tissue, but only after a selected period of time sufficient for the bioavailability of liberated cancer-specific antigens (monitored over the selected period of time) resulting from the necrosis or apoptosis to be at or near a maximum value. The present invention provides an alternative strategy to the ex vivo loading of target antigen to antigen presenting cells such as, for example, enriched autologous dendritic cells for purposes of enhancing an immune response.
Owner:CAVANAGH III WILLIAM ALOYSIUS +2
Determination of chemical or physical properties of sample or component of a sample
ActiveUS8010299B2Improve information qualitySmall sizeInterferometric spectrometrySpecial data processing applicationsSpectroscopyFourier transform on finite groups
The present invention offers an alternative strategy for the correlation of interference information to chemical and / or physical properties of a sample. This strategy can be implemented in a method and a system, which offer substantial technical and commercial advantages over state of the art techniques based on interference spectroscopy. The method comprises the steps of: a. obtaining an interferogram and / or at least one interferogram element corresponding to a modulation of electromagnetic signal emitted from, transmitted onto or through, or having interacted with at least a part of the sample, b. performing i. at least one transformation of the interferogram and / or a segment of the interferogram and / or an interferogram element with at least one function, ii. optionally repeating i) for another segment of the interferogram and / or interferogram element, wherein the transformation does not comprise a Fourier Transformation if i) is conducted only once, thereby obtaining at least one score, c. correlating said at least one score to the at least one chemical or physical property.
Owner:CHEMOMETEC AS
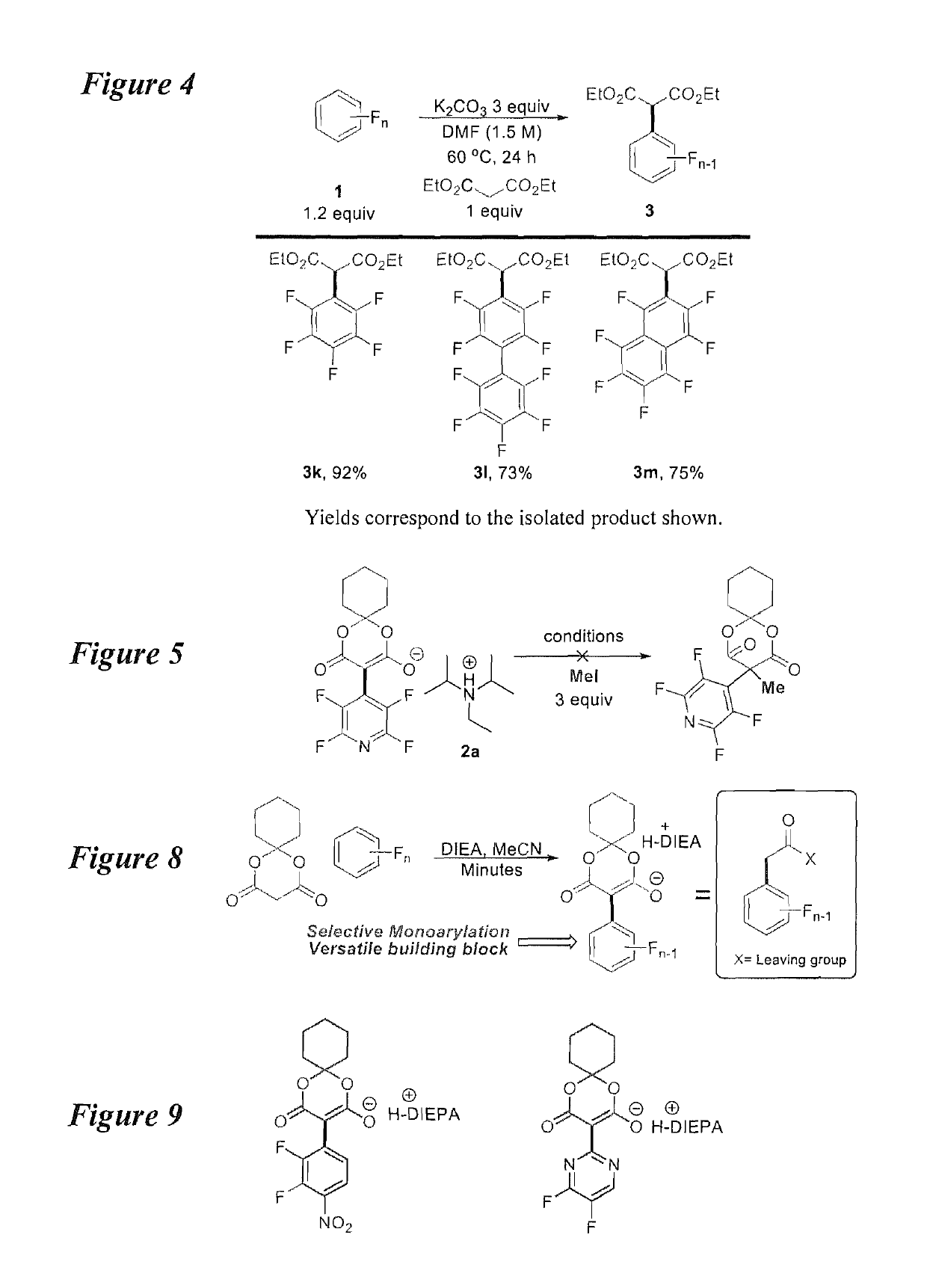
Facile and selective perfluoro-and polyfluoroarylation of meldrums acid
InactiveUS10316014B2Clean rapid operationally simple monoarylationEasy to functionalizeOrganic chemistryPhenyl acetic acidChromatographic separation
This disclosure relates generally to the facile and selective mono-perfluoro and -polyfluoroarylation of Meldrum's acid to generate a versatile synthon for highly fluorinated alpha-phenyl acetic acid derivatives which provide straightforward access to fluorinated building blocks. The reaction takes place quickly and all products were isolated without the need for chromatography. An embodiment provides an alternative strategy to access alpha-arylated Meldrum's acids which avoids the need for aryl-Pb(IV) salts or diaryliodonium salts and provides access to the tertiary product which was not previously synthetically accessible. The synthetic versatility and utility of the Meldrum's acid products is demonstrated by subjecting the products to several derivatizations of the Meldrum's acid products as well as photocatalytic hydrodefluorination which provide access to difficult but valuable synthetic targets such as multifluorinated aromatics.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Anti-sense microrna expression vectors
InactiveUS20110105592A1Inhibit expressionGenetic material ingredientsVertebrate cellsAlternative strategyMicroRNA
The present invention relates to an alternative strategy for expressing the antisense sequence of a miRNA. This system allows for continuous production of the antisense sequence and subsequently complete knockdown of the targeted miRNA.
Owner:RUTGERS THE STATE UNIV
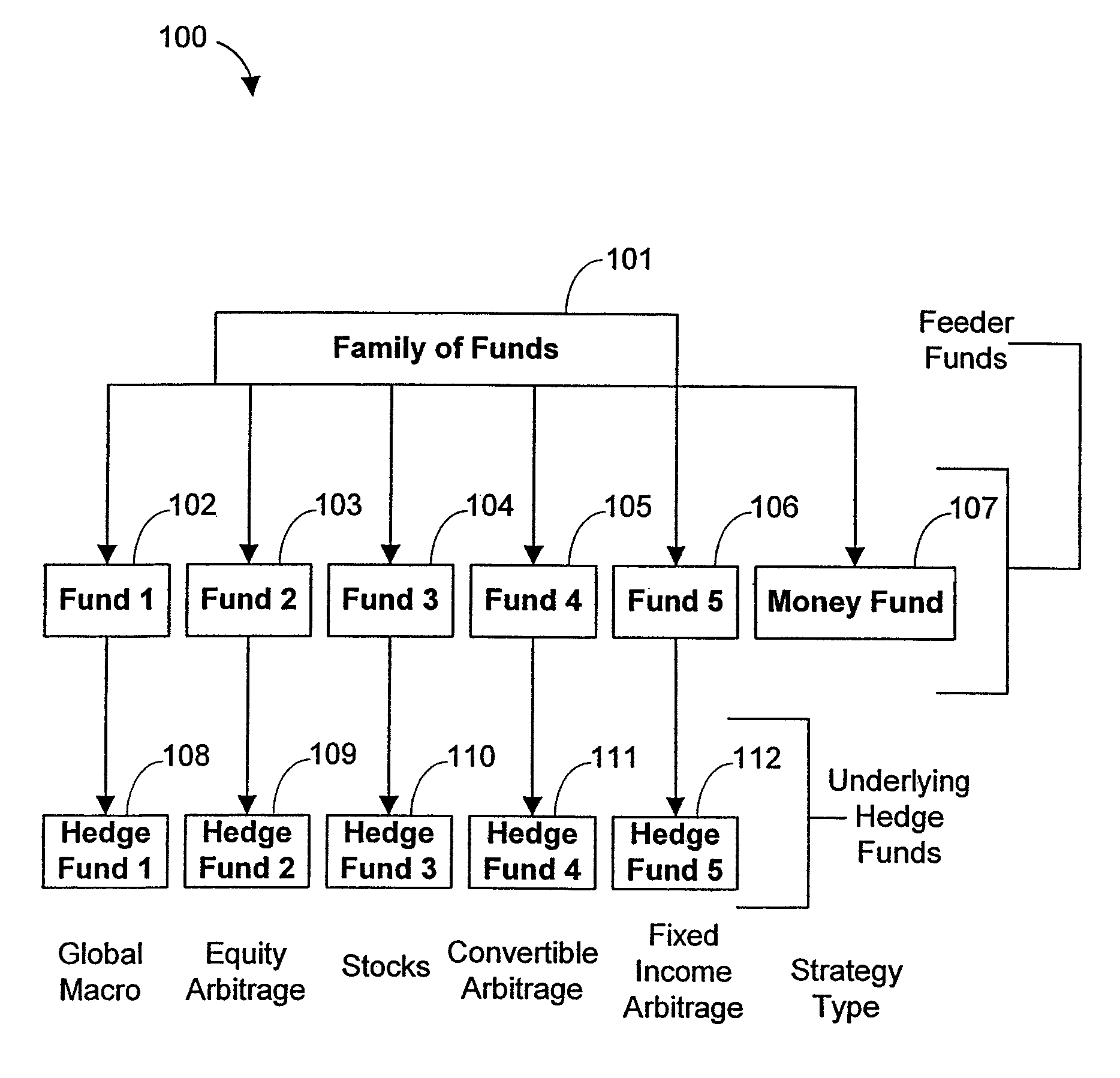
Systems and Methods for Offering and Servicing Hedge Funds
InactiveUS20110307416A1Improve liquidityRule out the possibilityFinanceCommerceAlternative strategyOperations research
A family of hedge funds, serving as “feeder funds” into underlying single-manager hedge funds, formed to provide smaller investors with the ability to allocate and reallocate assets among alternative strategies, and this basic structure combining interrelated systems and methods for offering, redeeming, exchanging, valuing, reporting and servicing the same is a new approach. The system and methods described herein provide investors having less than ultra-high net worth portfolios with access to hedge funds and the potential valuable diversification to an overall portfolio, and the ability to customize their portfolio of hedge funds to their individual needs and adjust such portfolio over time as seen fit in light of changing financial needs and market conditions. This invention gives a wide range of investors access to hedge funds, creating economic value using a new source of stable investor capital for hedge fund managers, a value shared with investors through reduced costs.
Owner:BANK OF AMERICA CORP
Systems and methods for offering and servicing hedge funds
InactiveUS8150756B2Improve liquidityRule out the possibilityFinanceCommerceAlternative strategyOperations research
A family of hedge funds, serving as “feeder funds”into underlying single-manager hedge funds, formed to provide smaller investors with the ability to allocate and reallocate assets among alternative strategies, and this basic structure combining interrelated systems and methods for offering, redeeming, exchanging, valuing, reporting and servicing the same is a new approach. The system and methods described herein provide investors having less than ultra-high net worth portfolios with access to hedge funds and the potential valuable diversification to an overall portfolio, and the ability to customize their portfolio of hedge funds to their individual needs and adjust such portfolio over time as seen fit in light of changing financial needs and market conditions. This invention gives a wide range of investors access to hedge funds, creating economic value using a new source of stable investor capital for hedge fund managers, a value shared with investors through reduced costs.
Owner:BANK OF AMERICA CORP
Application of dopamine receptor antagonist chlorprothixene in treatment of acute myeloid leukemia
ActiveCN110585197ADoes not affect normal physiological stateGrowth inhibitionOrganic active ingredientsAntineoplastic agentsApoptosisAlternative strategy
The invention discloses an application of a dopamine receptor antagonist chlorprothixene in treatment of acute myeloid leukemia, develops a new application of the dopamine receptor antagonist chlorprothixene as a drug, also provides a new way for treating acute myeloid leukemia, and uses the action mechanism between different fusion proteins and the existing APL theoretical model as support. Related in-vitro cell experiments and in-vivo animal experiments prove that: the chlorprothixene can induce cell apoptosis and autophagy pathways to promote death of AML cells, and can induce the decreaseof the expression quantity of fusion proteins PML-RARA and AML1-ETO, so that the chlorprothixene is a potential drug for inducing AML cell death, and provides a new visual angle for exploring alternative strategies of various AML subtype treatments.
Owner:SHANGHAI JIAO TONG UNIV +1
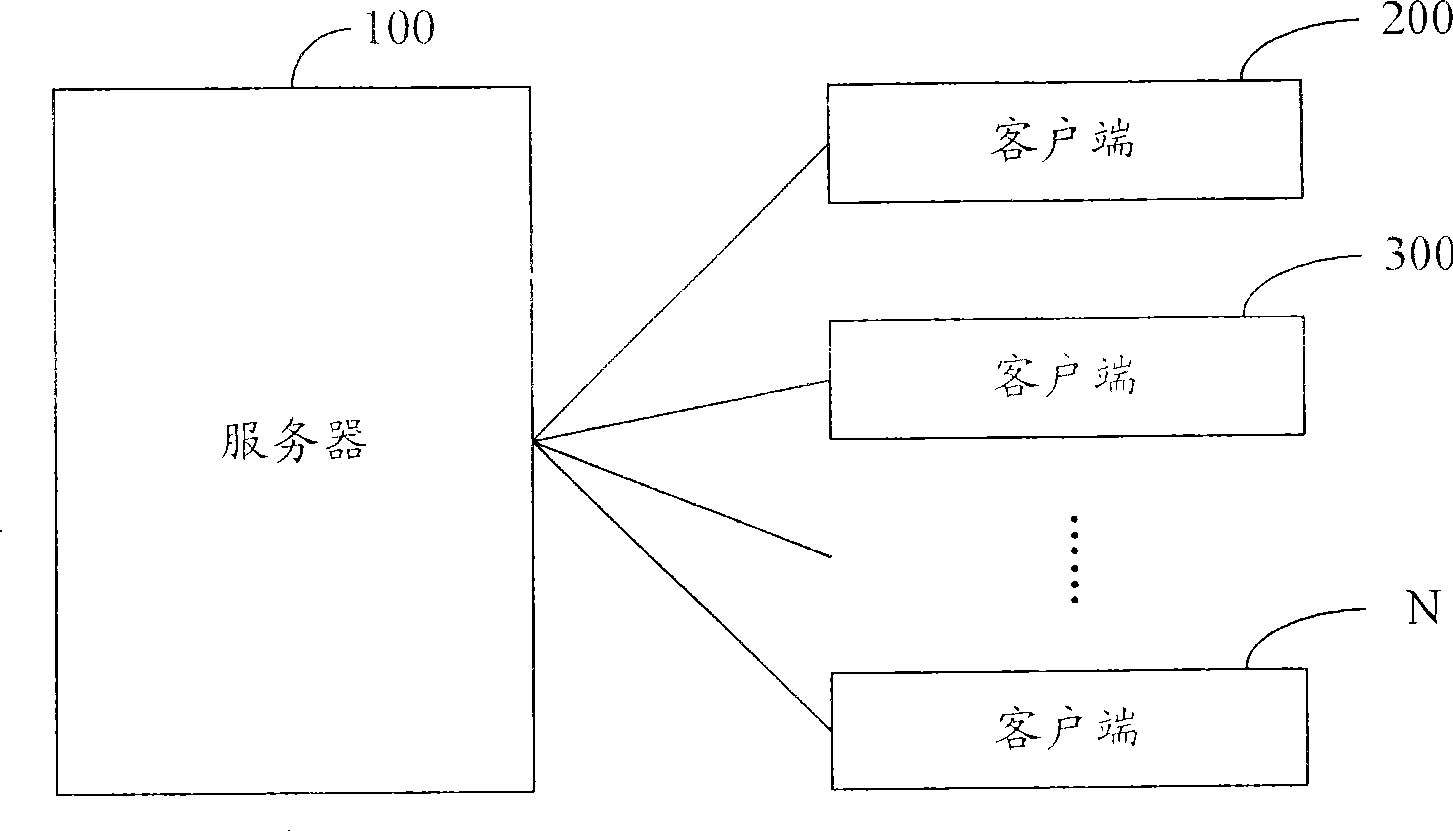
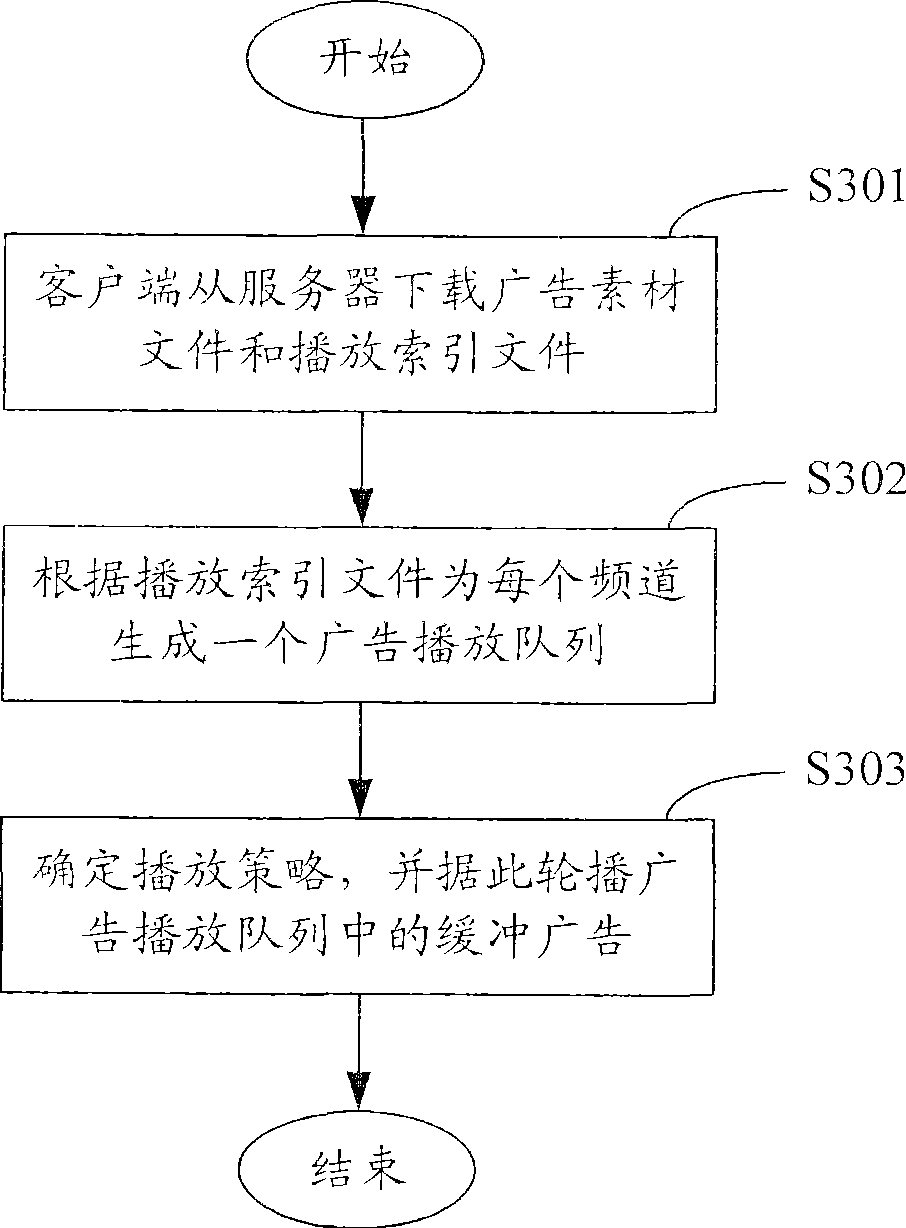
A method and system for broadcasting buffered advertisement in polling mode under the network living broadcast environment
ActiveCN100505630CIncrease flexibilitySpecial service provision for substationRecord information storagePersonalizationAlternative strategy
The invention relates to the communication field and provides a method and system for broadcasting buffered advertisements in rotation in a network live broadcast environment. The method includes the following steps: A. The server generates a play index file according to the personalized data reported by the client, the client downloads the creative material file and the play index file from the server, and the play index file includes the corresponding relationship between the buffer advertisement and the channel and Buffering the playback attributes of advertisements; B. The client generates an advertisement playback queue for each channel according to the playback index file; C. The client determines the playback strategy to determine the playback starting point and playback order of the advertisement playback queue, and according to the The playing strategy rotates the buffered advertisements in the advertisement playing queue within the buffering time. The present invention generates a playing index file in the server according to the personalized data reported by the client, and determines multiple optional playing strategies in the client, thereby improving the flexibility of the buffer advertisement playing process.
Owner:TENCENT TECH (SHENZHEN) CO LTD
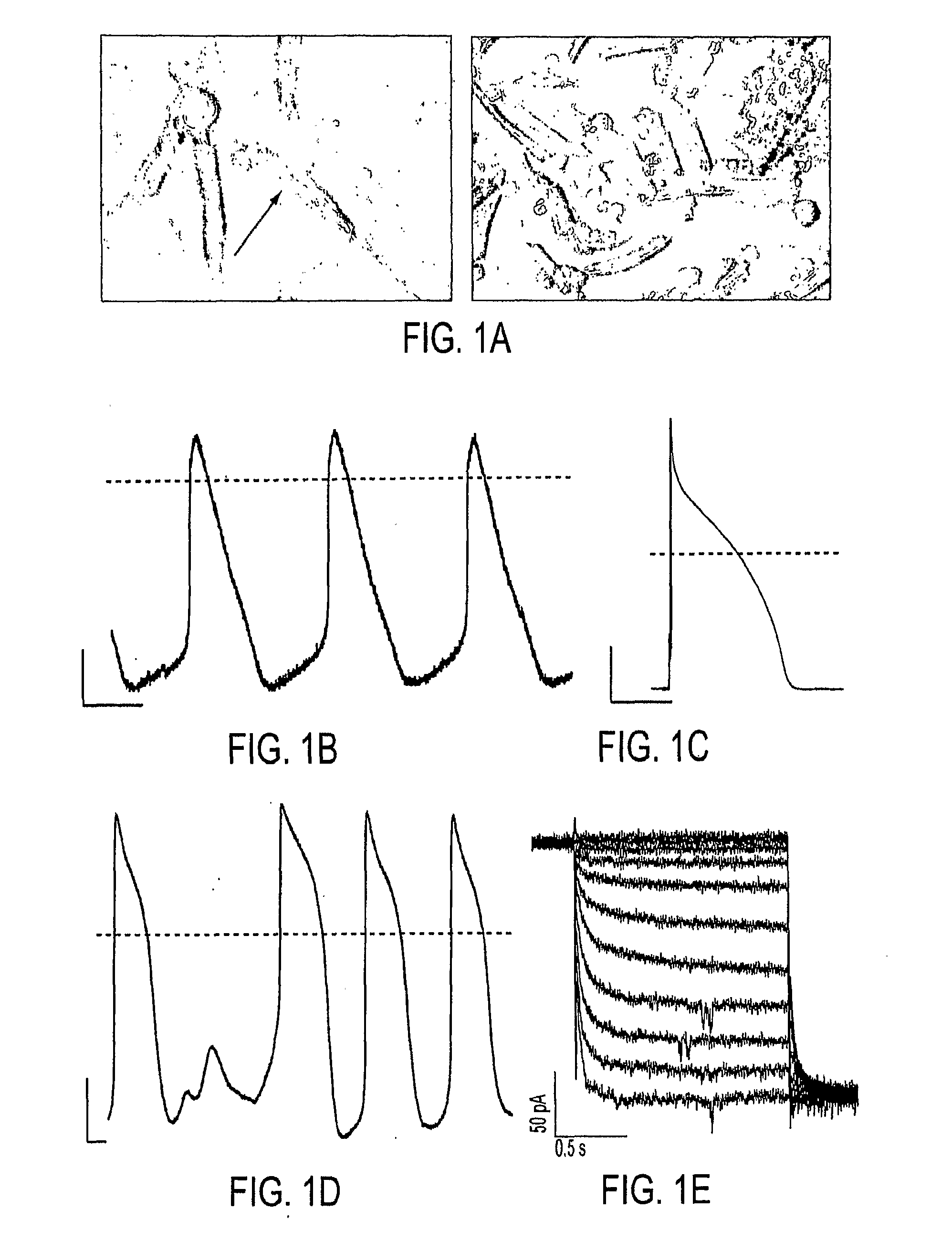
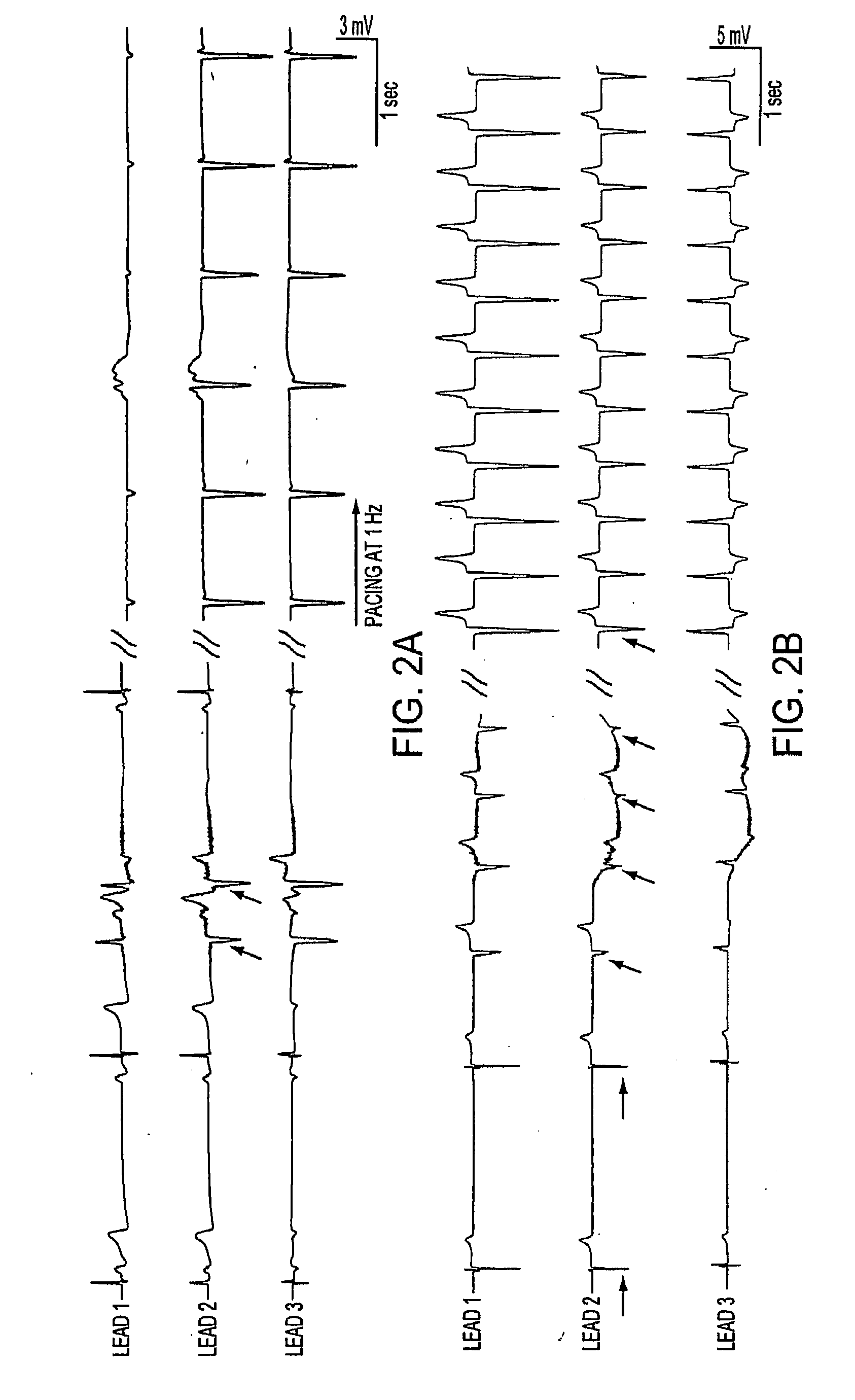
Biologically excitable cells
As an alternative strategy to electronic pacemaker devices, we explored the feasibility of converting normally-quiescent ventricular myocytes into pacemakers by somatic cell fusion. The idea is to create chemically-induced fusion between myocytes and syngeneic fibroblasts engineered to express HCN1 pacemaker ion channels (HCN1 fibroblasts), in normally-quiescent myocardium. HCN1-expressing fibroblasts formed stable heterokaryons with myocytes, generating spontaneously-oscillating action potentials as well as ventricular pacemaker activity in vivo and provides a platform for an autologous, non-viral, adult somatic cell therapy. We also converted a depolarization-activated potassium-selective channel, Kv1.4, into a hyperpolarization-activated non-selective channel by site-directed mutagenesis (R447N, L448A, and R453I in S4 and G528S in the pore). Gene transfer into ventricular myocardium demonstrated the ability of this construct to induce pacemaker activity, with spontaneous action potential oscillations in adult ventricular myocytes and idioventricular rhythms by in vivo electrocardiography. Given the sparse expression of Kv1 family channels in the human ventricle, gene transfer of a synthetic pacemaker channel based on the Kv1 family has therapeutic utility as a biological alternative to electronic pacemakers.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
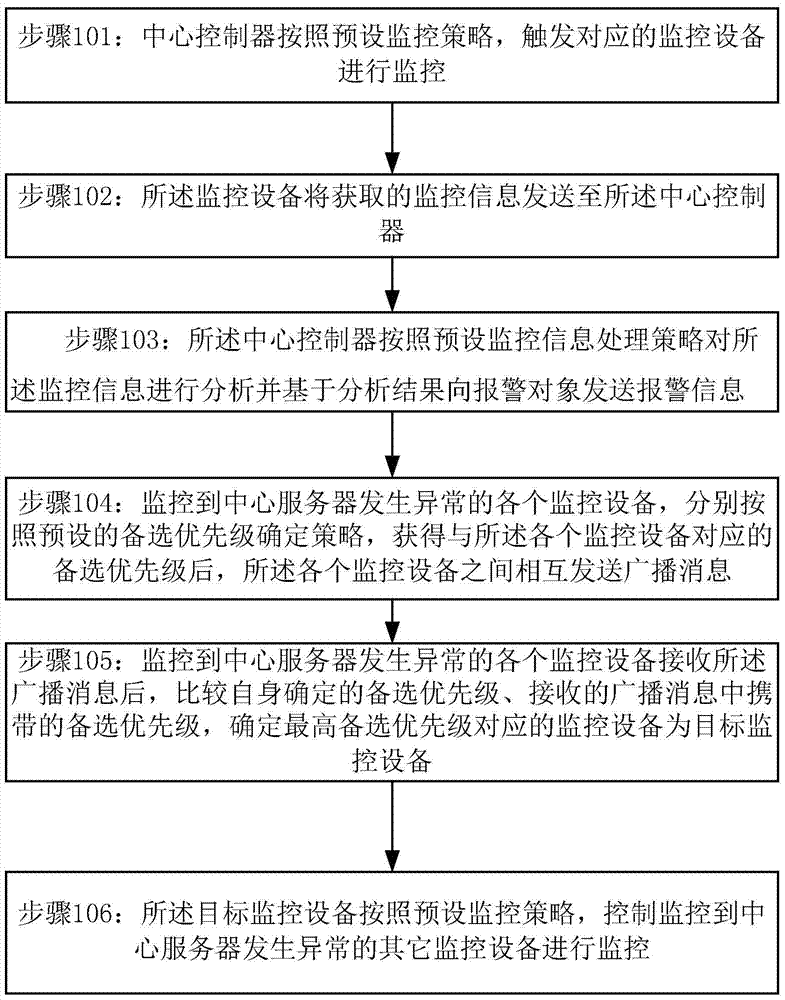
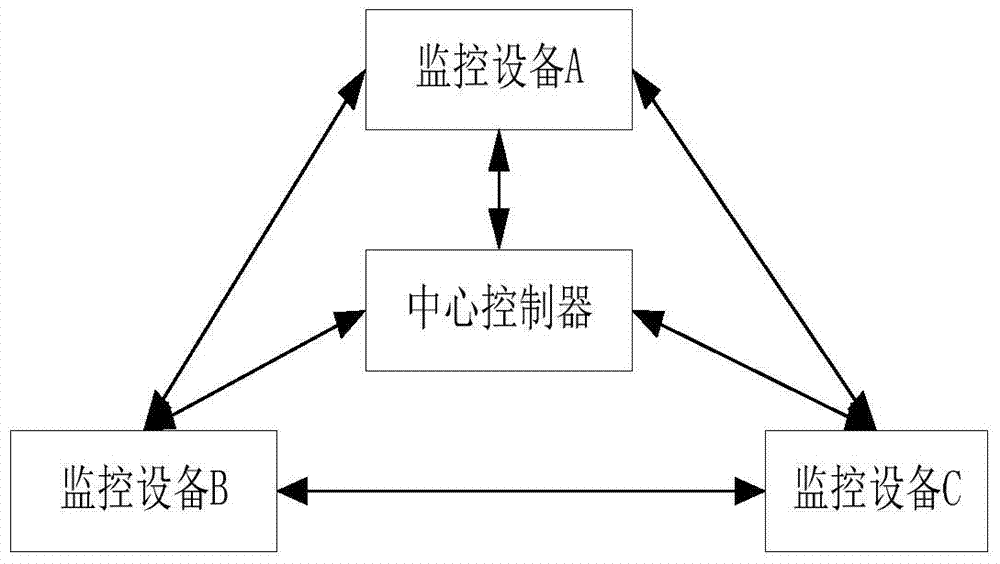
A monitoring management method and system based on a monitoring system
ActiveCN104574876BImprove securityCapable of self-constructionMultiple digital computer combinationsAlarmsAlternative strategyMonitoring system
Owner:EQUES TECH
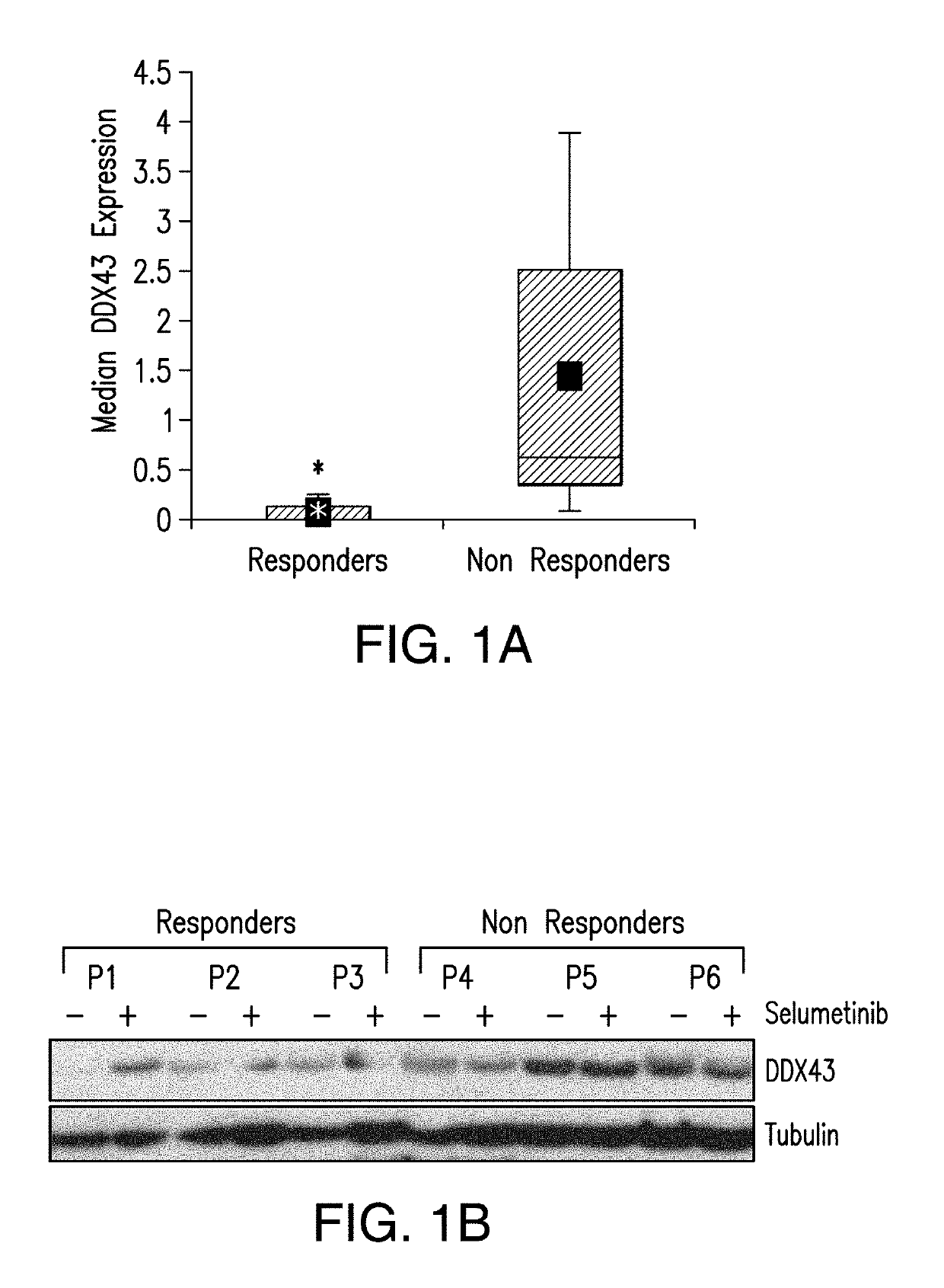
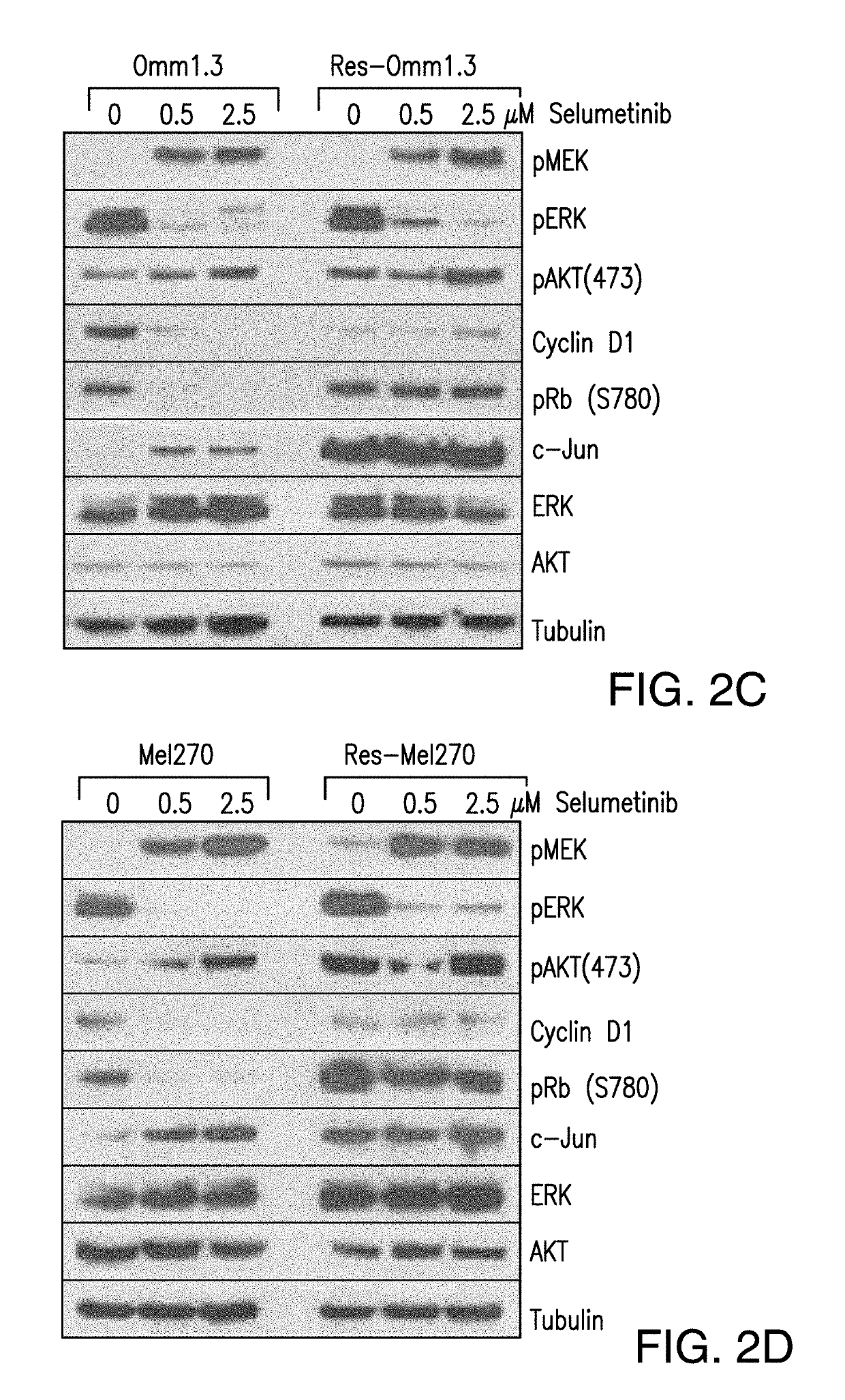
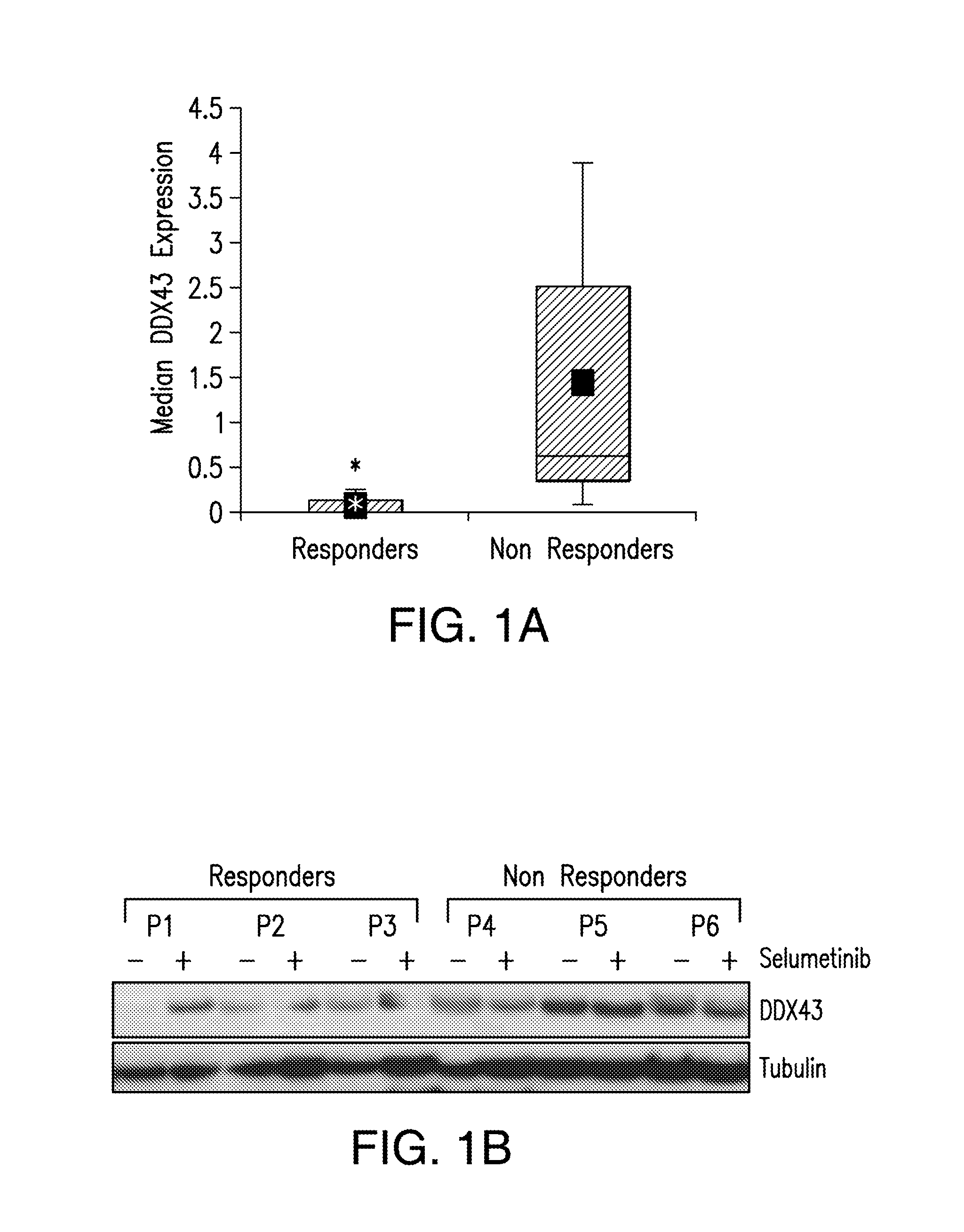
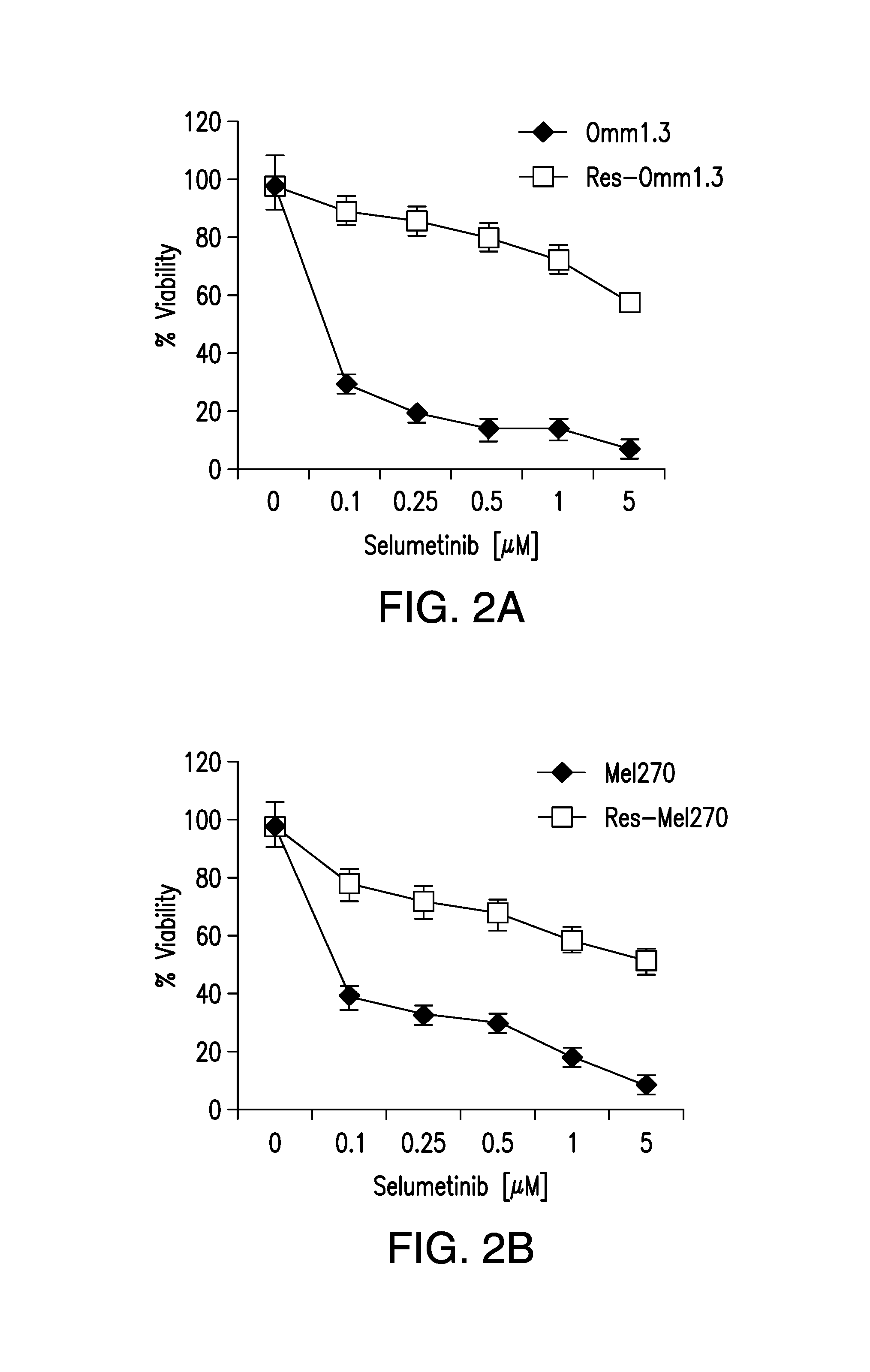
DDX43 as a biomarker of resistance to MEK1/2 inhibitors
InactiveUS10400285B2Organic active ingredientsMicrobiological testing/measurementAcquired resistanceHigh level expression
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Mobile video order service system with optimized performance and realizing method
InactiveCN100388788CImprove performancePerformance improvement or enhancementTwo-way working systemsTransmissionAlternative strategyCache hit rate
The mobile VOD business system with super performance based on GPRS mobile network comprises: besides traditional net element, a CCCP server on boundary of GGSN and PDN to release load for SCS and reduce bandwidth consumption between GGSN and SGS, a UECP server arranged in BSS that is between BSC and BTS to self-adaptive improve buffer hit rate based on buffer alternative strategy and ensure fast response to most user request, and a high-speed IP DL between CCP and UECP to transmit flow media content and construct two-stage buffer device with two proxy servers to bypass great media and reduce data flux and load for other link and eliminate the effect of VOD to network performance.
Owner:BEIJING UNIV OF POSTS & TELECOMM
A dynamic strategy simulation and optimization method based on microgrid
ActiveCN108288855BImprove adaptabilityImprove reliabilitySingle network parallel feeding arrangementsAc network load balancingControl engineeringGenetics algorithms
Owner:GUODIAN NANJING AUTOMATION
Anti-sense microrna expression vectors
The present invention relates to an alternative strategy for expressing the antisense sequence of a miRNA. This system allows for continuous production of the antisense sequence and subsequently complete knockdown of the targeted miRNA.
Owner:RUTGERS THE STATE UNIV
Confrontation simulation optimizing method and system
ActiveCN104699983AGood strategyEfficient anti-simulation optimization methodSpecial data processing applicationsAlternative strategySimulation
The invention provides a confrontation simulation optimizing method. The confrontation simulation optimizing method comprises the following steps of S1, sending alternative strategies and confrontation conditions to a plurality of simulation platforms to be simulated to obtain a plurality of confrontation results of a preset interference strategy under the confrontation conditions; S2, processing a plurality of confrontation results to obtain an optimal strategy; S3 judging whether the optimal strategy satisfies a preset condition or not, enabling the optimal strategy to be served as a result to be output if yes and repeatedly executing S1 to S3 if no. The confrontation simulation optimizing method is efficient and allows parallel execution. The invention also provides a confrontation simulation optimizing system.
Owner:TSINGHUA UNIV
Ddx43 as a biomarker of resistance to mek1/2 inhibitors
InactiveUS20160258027A1Organic active ingredientsMicrobiological testing/measurementAcquired resistanceHigh level expression
The present invention relates to methods and compositions for determining the likelihood that a subject suffering from a cancer will benefit from treatment with a MEK inhibitor. It also relates to methods of treatment based on such determination. The invention is based, at least in part, on the discoveries that DDX43 mRNA and protein are expressed at high levels in biopsies from “non-responder” UM patients and that selumetinib-resistant cell lines showed high DDX43 expression which correlated with increased expression and activity of RAS. It was found that KRAS and HRAS but not NRAS, mediated expression of pERK and pAKT, bypassing oncogenic GNAQ. The invention is further based on the discovery that selumetinib-resistant cells became sensitive to AKT inhibition, suggesting alternative strategies for the treatment of cancer patients with acquired resistance to MEK inhibitors.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com