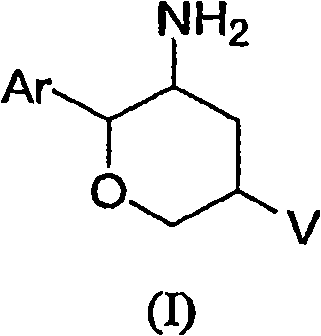
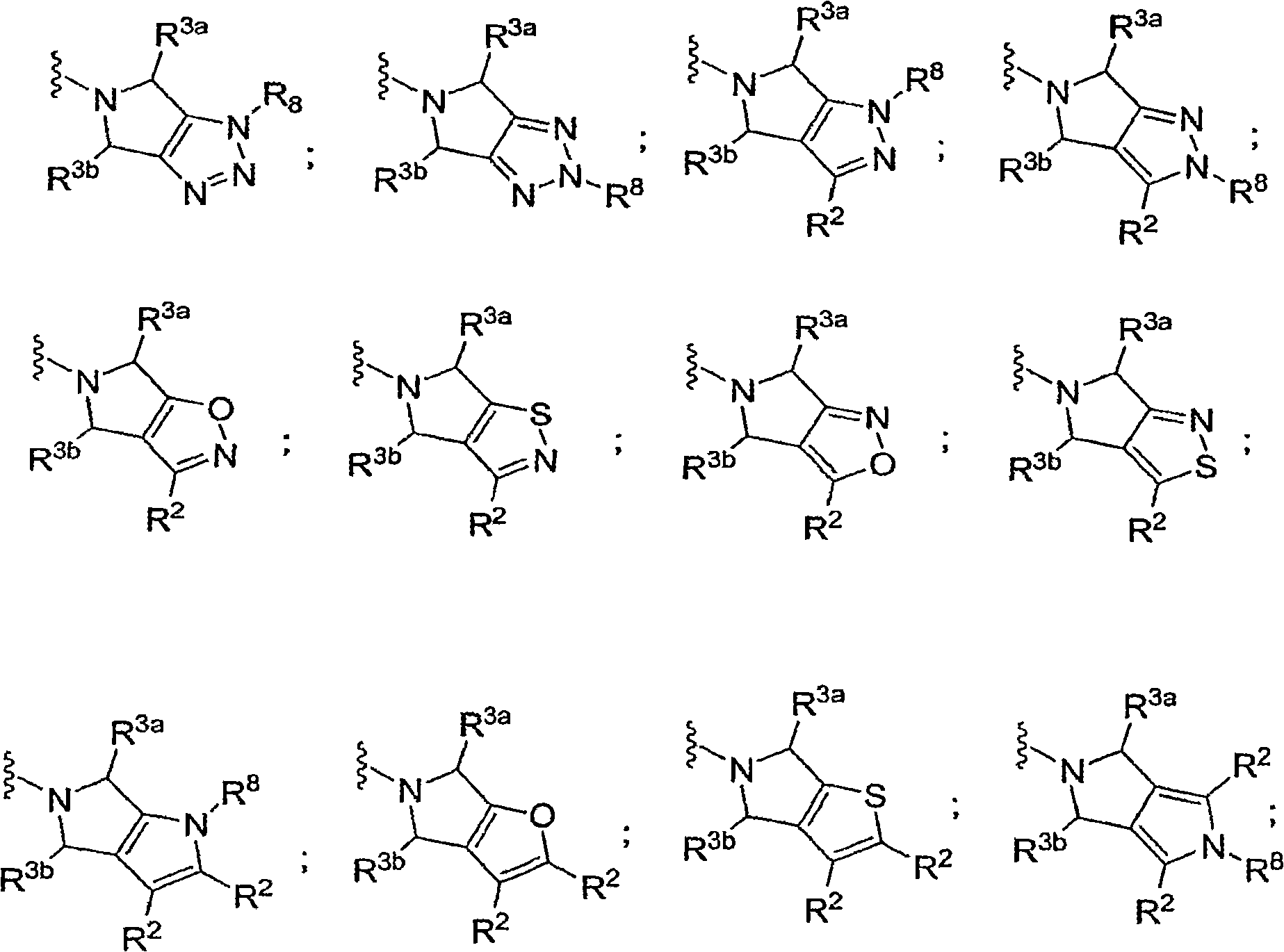
Aminotetrahydropyrans as dipeptidyl peptidase-IV inhibitors for the treatment or prevention of diabetes
An alkyl and hydroxyl technology, applied in the field of type 2 diabetes, can solve problems such as extensive research on DPP-4 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277]
[0278] (2R, 3S, 5R)-5-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-2-(2,4,5 -Trifluorophenyl)tetrahydro-2H-pyran-3-amine dihydrochloride
[0279] Step A: [(2R,3S,5R)-5-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-2-(2 , 4,5-trifluorophenyl)tetrahydro-2H-pyran-3-ylcarbamate tert-butyl ester
[0280] To a stirred solution of Intermediate 1 (50 mg, 0.145 mmol) and Intermediate 4 (29 mg, 0.235 mmol) in methanol (1.0 mL) was added decaborane (6 mg, 0.048 mmol), and the mixture was stirred for 15 hours and under reduced pressure. Evaporate under pressure. The product (diastereomer with poor TLC mobility) was purified by preparative thin layer chromatography using 5% methanol in dichloromethane to give [(2R,3S,5S)-5-(1 -Methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-2-(2,4,5-trifluorophenyl)tetrahydro-2H-pyridine tert-butyl pyran-3-ylcarbamate. LC-MS 453.17(M+1).
[0281] Step B: (2R,3S,5R)-5-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-2-...
Embodiment 2
[0284]
[0285] (2R, 3S, 5R)-5-(1-methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-2-(2,4,5 -Trifluorophenyl)tetrahydro-2H-pyran-3-amine dihydrochloride
[0286] This compound was prepared in a similar manner to Example 1 Step B, but using the diastereomer [(2R, 3S, 5R)-5-(1 -Methyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5(1H)-yl)-2-(2,4,5-trifluorophenyl)tetrahydro-2H-pyridine tert-butyl pyran-3-ylcarbamate. 1 H NMR (500MHz, CD 3 OD): δ8.14-8.05(m, 1H); 7.41(s, 1H); 7.31-7.24(m, 1H); 4.96-4.82(m, 4H); 4.52(d, 1H, J=16Hz); 4.20(s, 1H); 4.14-4.04(m, 2H); 3.90(s, 3H); 3.33-3.28(m, 1H); 2.83(d, 1H, J=16Hz); 2.41(t, 1H, J =16Hz); LC-MS 353.17(M+1).
Embodiment 3
[0288]
[0289] (2R,3S,5R)-2-(2,5-difluorophenyl)tetrahydro)-5-(4,6-dihydropyrrolo[3,4-c]pyrazole-5(1H)- base) tetrahydro-2H-pyran-3-amine dihydrochloride
[0290] Step A: [(2R,3S,5R)-2-(2,5-Difluorophenyl)-5-(4,6-dihydropyrrolo[3,4-c]pyrazole-5(1H) -yl)-)tert-butyl tetrahydro-2H-pyran-3-ylcarbamate
[0291] A solution of Intermediate 2 (6.03 g, 18.4 mmol) and Intermediate 3 (2.61 g, 23.94 mmol) in methanol (135 mL) was stirred for 30 minutes and then treated with decaborane (674 mg, 5.53 mmol). The mixture was stirred overnight and evaporated under reduced pressure. The resulting residue is in (Silicon dioxide, with ethyl acetate (6L), 10% ammonia ethyl acetate solution (2L) containing 5-6% ethanol, 10% ammonia ethyl acetate solution (3L) containing 8-12% ethanol Purification on (sequential elution) gave the title compound as the less mobile diastereomer (silica, TLC in 6% methanol in dichloromethane containing 10% ammonia). 1 H NMR (500MHz, CD 3 OD): δ1.18-1.24(m, 9H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com