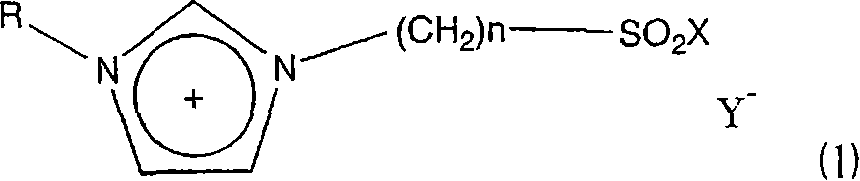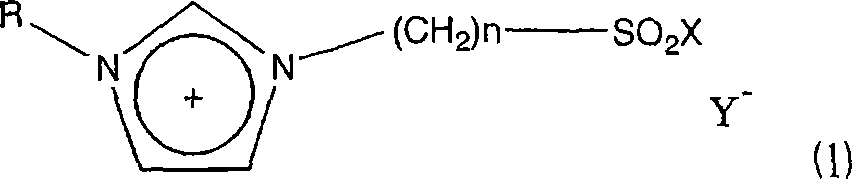Ionic liquid and method of reaction using the same
An ionic liquid, reaction technology, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve problems such as sufficient satisfaction and difficulty in regeneration of catalyst systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] 1-Methylimidazole and 1,4-butane sultone were mixed with stirring at a molar ratio of 1:1, and stirred at room temperature for 24 hours to perform a reaction. At this time, white crystals were obtained in a yield of 100%. Next, after crushing the white crystals, they were washed several times with ether, and the washed crystals and CF were mixed at a molar ratio of 1:1. 3 SO 3 H, reacted at 60°C for 24 hours to obtain the ionic liquid 4a in which n=4 and X=OH in the formula (2). Next, thionyl chloride was added little by little to the ionic liquid under reflux (about 80°C) for reaction to obtain ionic liquid 4b in which n=4 and X=Cl in formula (2).
[0104] A more favorable method for obtaining the ionic liquid 4b from the ionic liquid 4a is shown below.
[0105]0.12 mol of thionyl chloride was put into a 50 ml two-ended flask, and the mixture was refluxed while stirring with an electromagnetic stirrer. Under reflux conditions, 0.1 mol of the above-mentioned ionic l...
Embodiment 2
[0107] Ionic liquids 3a, 4a, 3b and 4b were used. For comparison, while studying the BMImBF of the well-known ionic liquid 4 and BMImPF 6 . In the experiment, the ionic liquid, p-xylene (30 mmol) and styrene were placed in a 10 ml test tube equipped with an electromagnetic stirrer, and the reaction was carried out at 70° C. for 1 to 5 hours. The molar ratio of styrene to ionic liquid reaches 10, and the molar ratio of aromatic hydrocarbon to styrene reaches 9:1-3:1. After the reaction, the upper organic layer was separated and analyzed by FID gas chromatography (Shimadzu GC-14A, ULBON HR-52 capillary column 25 mm×0.32 mm).
[0108] In Table 1 are shown the results of the Friedel-Crafts-alkylation reaction of p-xylene from styrene under various reaction conditions. In all of these cases, 2 main products were detected, monostyrenated and distyrenated. All of them are industrially desired substances. It was found that when p-xylene and styrene were reacted using Lewis acidi...
Embodiment 3
[0116] The alkylation reaction was carried out in the same manner as in Example 1, using the ionic liquid 4a as the catalyst, changing the types of aromatic hydrocarbons and alkenes. The kinds of aromatic hydrocarbons and alkenes and the reaction time are shown in Table 2 together with the results. For other conditions, the reaction temperature is 70° C., 30 mmol of aromatic hydrocarbon, aromatic hydrocarbon / alkene=3 (molar ratio), and alkene / ionic liquid=10 (molar ratio). The reaction conditions and results are shown in Table 2.
[0117] experiment
[0118] In general, the ionic liquids of the present invention are also effective catalysts for the alkylation of benzene or toluene by styrene. It can be speculated that benzene can be styrenated more easily than toluene or p-xylene under the same conditions. However, it is very interesting that even if the acidic ionic liquid catalyst 4a is applied to the alkylation reaction of long-chain alkenes like benzene and hex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com