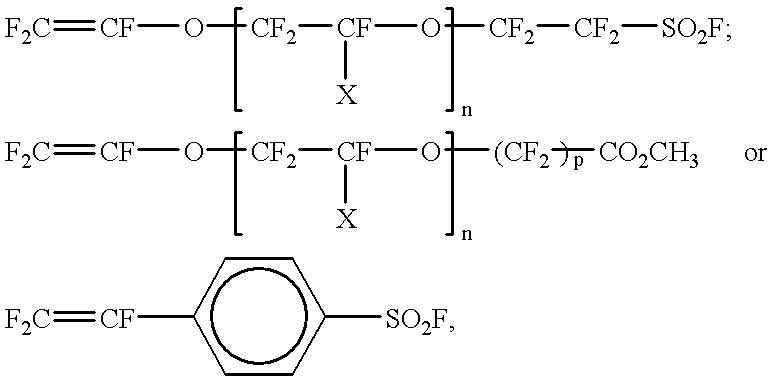
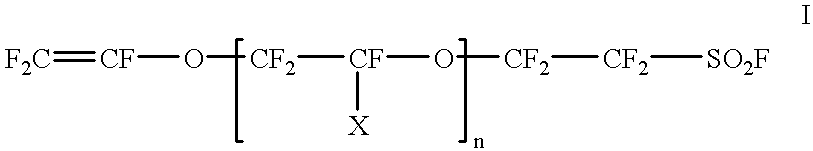
Cross-linked sulphonated polymers and method for preparing same
a sulphonated polymer and crosslinked technology, applied in the field of crosslinked sulphonated polymers and methods for preparing same, can solve the problems of tetrafluoroethylene (tfe) being a hazardous product to handle, unable to meet the requirements of a wide range of applications, and not having good mechanical properties, etc., to achieve the effect of improving mechanical properties, less homogeneous, and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0051] A commercial membrane of Nafion 117.RTM. of 175 .mu.m thickness in the form of a lithium salt is dried and cut in slices of 4 cm.times.10 cm. The membrane spiraly rolled up is treated with 2 g of sulfur dimethylaminotrifluoride (CH.sub.3).sub.2NSF.sub.3 in 50 ml of THF under reflux, then rinsed. The polymer now containing sulfonated groups in the form SO.sub.2F is immersed in a solution of 60 mg of the hexamethydisilazane sodium salt in 20 ml of anhydrous diglyme and refluxed under argon. After 3 hours, the membrane is removed from the reaction media, rinsed with THF and treated with a solution of 500 mg of sodium trimethysilanoate in the same solvent. After 48 hours, the membrane is washed with water and ethanol, and transformed in the hydronium salt by several successive immersions in a nitric acid solution 2 M in water at 60.degree. C. High resolution solid NMR shows that 32% of the sulfonyl group of the membrane are in the sulfonimide form and 78% in the sulfonate form. T...
example 3
[0052] A copolymer of tetrafluoroethylene in perfluorovinyloxyethane-sulfo-nyle fluoride containing 35% molar of the sulfonated monomer is heat calendered to form a 20 microns thick film. The compound [Na(Si(CH.sub.3).sub.3NSO.sub.2CF.sub.2].sub.2CF.sub.2 is prepared from the hexafluoropropane-1,3-disulfonic acid fluoride according to the following sequence of reactions:
[FSO.sub.2CF.sub.2].sub.2CF.sub.2+6 NH.sub.3.fwdarw.2 NH.sub.4F+[(NH.sub.4)HNSO.sub.2CF.sub.2].sub.2
[(NH.sub.4)HNSO.sub.2CF.sub.2].sub.2+Na.sub.2CO.sub.3.fwdarw.[(Na)HNSO.sub-.2CF.sub.2].sub.2+2 NH.sub.3+H.sub.2O+CO.sub.2
[(Na)HNSO.sub.2CF.sub.2].sub.2+HN[(Si(CH.sub.3).sub.3].sub.2.fwdarw.Na[Si(-CH.sub.3).sub.3NSO.sub.2CF.sub.2].sub.2CF.sub.2+NH.sub.3
[0053] 10 square sections of 10 cm.times.10 cm of this membrane separated by polypropylene wire-mesh, are immersed in a glass recipient and covered with a solution of 600 mg of the sulfamide disodic derivative in 50 ml in diglyme. The mixture is heated to 125.degree. C. f...
example 4
[0055] A membrane of 20 .mu.m of the copolymer of tetrafluoroethylene and perfluorovinyloxyethanesulfonyl fluoride of Example 3 is treated in a solution of 800 mg of the sulfamide disodic derivative of Example 3 and 400 mg of sodium trimethylsilanoate in 50 ml of diglyme. The mixture is heated at 125.degree. C. for 4 hours under argon. The membrane is removed and washed with deionized water and exchanged with protons as described in Example 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com