Preparation method of 2-hydroxyl-1-{4-(2-hydroxyethyl) phenyl}-2-methyl-1-acetone
A technology of hydroxyethoxy and hydroxyl, applied in the preparation of carbon-based compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of high corrosion, troublesome post-processing, slow reaction, etc., and simplify the process flow , Reduce production cost, improve the effect of yield
Inactive Publication Date: 2010-08-25
GANSU JINDUN CHEM
View PDF2 Cites 19 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
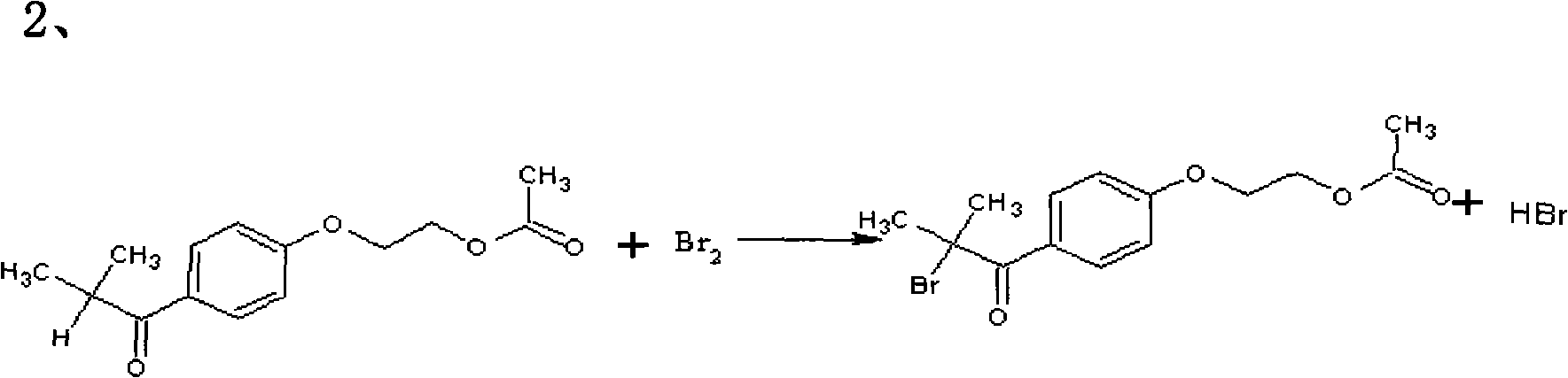
The main disadvantage of this process is that the bromination reaction uses glacial acetic acid as a solvent, which is highly corrosive to the equipment, and the reaction is relatively slow, requiring more than 10 hours
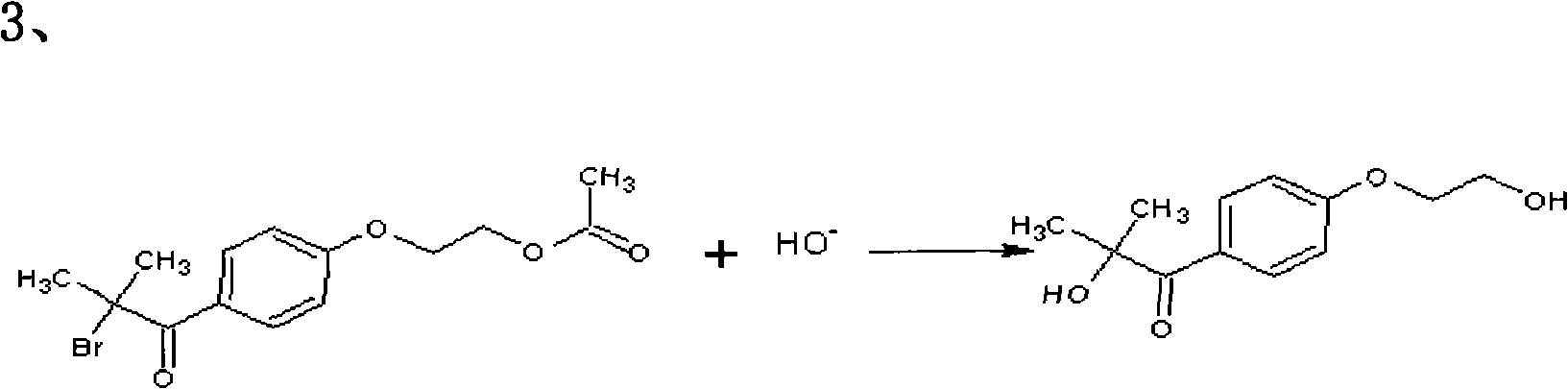
The hydrolysis process uses a large amount of ethanol as a solvent, and the post-treatment is cumbersome and costly
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
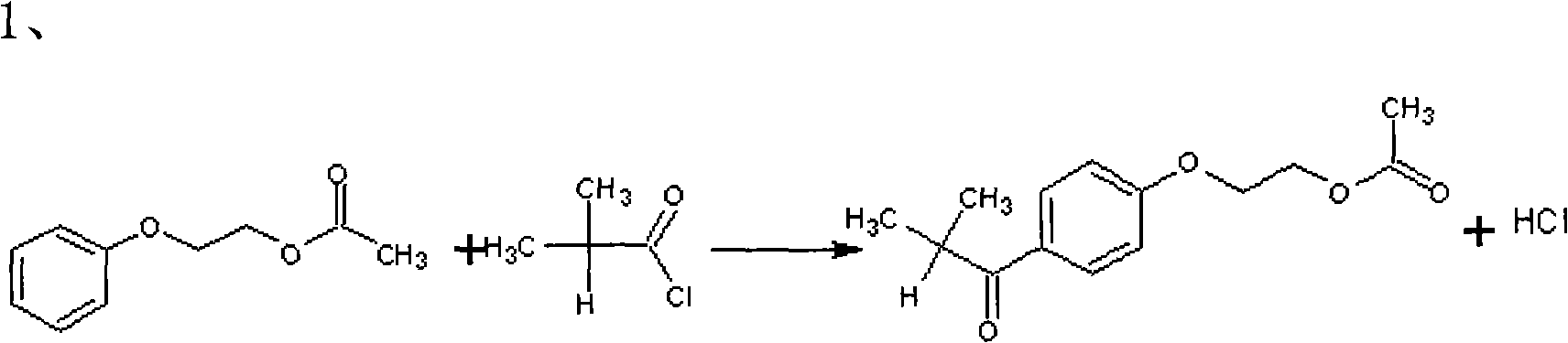
The invention relates to a preparation method of 2-hydroxyl-1-{4-(2-hydroxyethyl) phenyl}-2-methyl-1-acetone, which comprises the following steps that: ethylene glycol phenyl ether acetate serves as the raw material and carries out friedel-crafts reaction with isobutyl chloride with the molar ratio of 1 to 1.2 times of equivalent under the catalysis of lewis acid, the reaction temperature is -5 to 5DEG C, and the reaction time is 3 to 6h; bromination is carried out under the catalysis of N, N-dimethylformamide or iodine; at room temperature, phase transfer catalyst is used for carrying out catalysis and hydrolysis to a brominated product; and finally the product is prepared through purification and crystallization. The preparation method of 2-hydroxyl-1-{4-(2-hydroxyethyl) phenyl}-2-methyl-1-acetone adopts dichloromethane or dichloroethane as the solvent during the bromination process, does not use acetic acid any more, carries out reaction under the catalysis of DMF or I2, so that the reaction time is greatly shortened. The phase transfer catalyst is introduced during the hydrolysis process, so that the intermediate brominated product directly has reaction with alkali in water, thereby preventing using a large amount of ethanol as the solvent, simplifying the process, reducing the production cost and improving the yield.
Description
technical field The invention relates to the technical field of organic chemistry, and relates to a method for synthesizing a photoinitiator 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone. Background technique 2-Hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone is a UV curing initiator, which is the only photoinitiator allowed by the FDA certification system, available In adhesives that are not in direct contact with food, low odor, low volatility, low yellowing, with active hydroxyethoxyl terminal hydroxyl groups, can participate in the reaction. It can be used in water-based light-curing systems with high melting point, and can also be used in UV-curable powder coatings. At present, the common method of synthesizing 2-hydroxyl-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone is: ethylene glycol phenyl ether acetate and isobutyryl chloride in Lewis acid The Friedel-Crafts reaction occurs under catalysis, and the product is brominated in the solven...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C49/84C07C45/65C07C45/64
Inventor 曹新刚程金奎张莉
Owner GANSU JINDUN CHEM
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com