Preparation method for asymmetric synthesis of pregabalin
A pregabalin, asymmetric technology, applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation and other directions, to achieve the effects of improved product yield, novel synthesis route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Preparation of Compound 9
[0063] At room temperature, add 11.3 g of isovaleraldehyde, 50 ml of n-hexane, 0.75 g of acetic acid, and 0.63 g of di(n-)propylamine into a dry 100 mL single-necked flask. Stir well and add 20.0 g of diethyl malonate. Raise the temperature and reflux, connect the water separator to divide the water until no water is produced. Stop heating and stirring, cool to room temperature naturally, add 25mL water to wash, separate the organic phase, wash once with 20mL 1mol / L NaOH aqueous solution, and finally wash with 5% NH 4 The Cl aqueous solution was washed once, and the organic phase was concentrated to obtain the residue as a yellow liquid, and 25.7 g of the target product was distilled out by rectification. Yield: 86.7%.
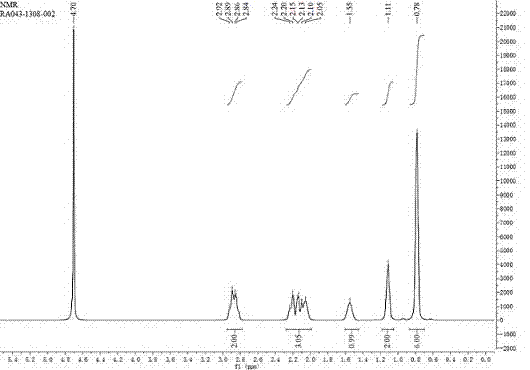
[0064] 1 HNMR(400MHz,CDCl3): 6.99-7.02(t, 1H, -C=C H ), 4.27-4.32(q, 2H, -OC H 2 CH 3 ), 4.20-4.25(q, 2H, -OC H 2 CH 3 ), 2.17-2.20(t, 2H, -C H 2 C=CH), 1.78-1.84(m, 1H, -C H (CH 3 ) 2 ), 1.25-1...
Embodiment 2
[0065] Example 2 Preparation of Compound 9
[0066] At room temperature, add 11.3g of isovaleraldehyde, 100ml of toluene, 0.75g of acetic acid, and 1.40g of N-methylpiperazine into a 250 mL reaction bottle equipped with a water separator. After stirring evenly, add the material diethyl malonate Esters 20.0 g. Heat up and reflux, connect the water separator to divide the water until no water is produced, and separate the water. Stop heating and stirring, cool to room temperature naturally, add 25mL water to wash, separate the organic phase, wash once with 20mL 1mol / L NaOH aqueous solution, and finally wash with 5% NH 4 The Cl aqueous solution was washed once, the toluene layer was dried with anhydrous magnesium sulfate, the desiccant was filtered off, and the residue was concentrated to obtain a yellow liquid, and 28.0 g of the target product was distilled out by rectification. Yield: 94.4%.
[0067] The 1H NMR spectrum is the same as above.
Embodiment 3
[0068] Example 3 Preparation of Compound 34
[0069] At room temperature, add compound 4 (20.0 g) and nitromethane 26.8 g into a dry 100 mL single-necked flask, stir evenly, cool down to -5°C-0°C, slowly add nitromethane containing DHQ-PYR 4.1 g dropwise 10.0 mL of solvent was added dropwise within 30 minutes, and stirring was continued for 1 hour at -5°C-0°C. The ice bath was removed, and the reaction was stirred at 25-30°C. TLC detected that the starting material disappeared. Add 30mL of 2mol / L hydrochloric acid aqueous solution, under stirring, add 20mL of saturated sodium chloride solution, extract three times with 40mL of ethyl acetate, combine the ethyl acetate layer, wash twice with 10mL of saturated sodium carbonate solution, and then wash twice with 10mL of water , 20.4 g of light yellow oily liquid distilled from the ethyl acetate layer under reduced pressure. Yield: 80.6%, ee value 99.4%, purity 98.3%.
[0070] 1 HNMR(400MHz, CDCl3,):4.50-4.54(q, 1H, -C H 2 NO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com