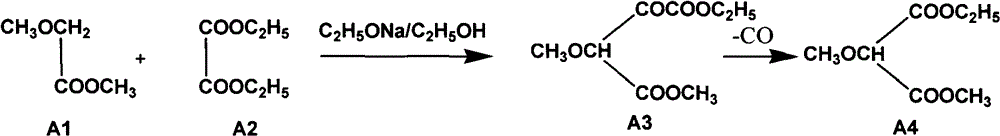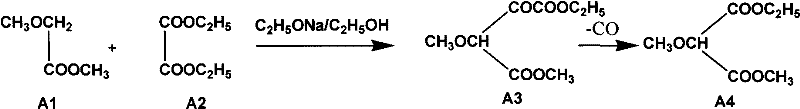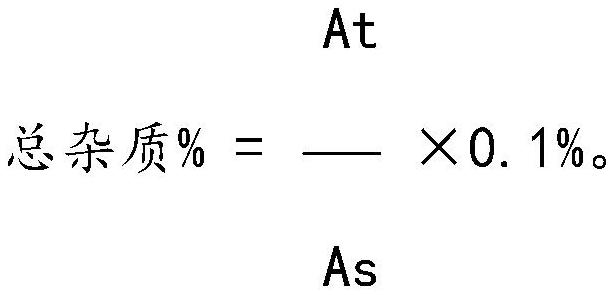Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Sulfadoxine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
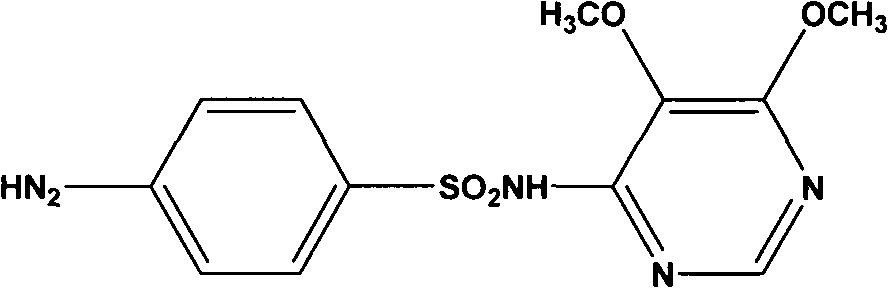
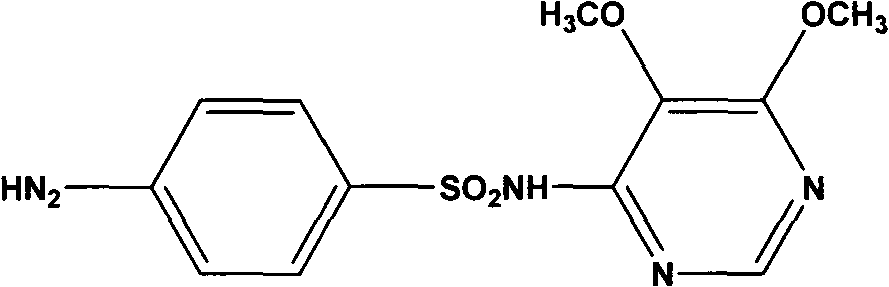
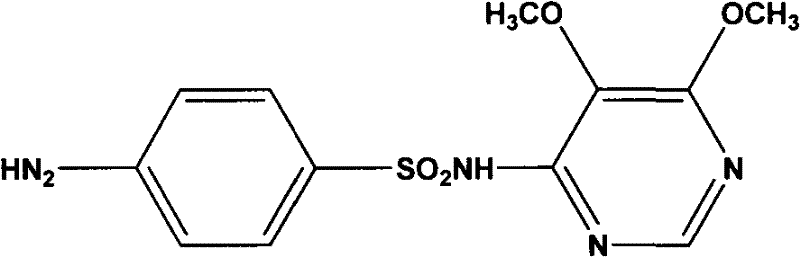
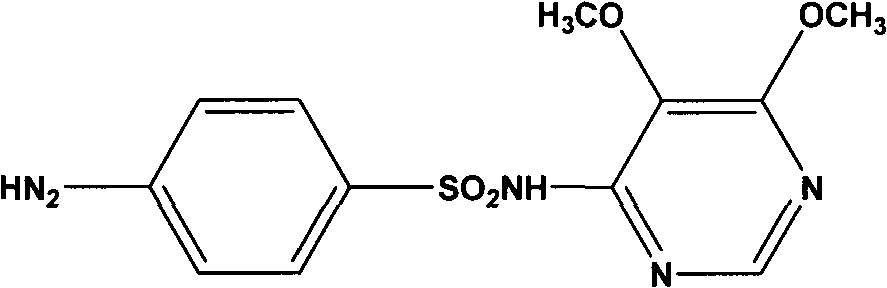
Sulfadoxine (also spelled sulphadoxine) is an ultra-long-lasting sulfonamide used in combination with pyrimethamine to treat malaria. It was previously used to prevent malaria but due to high levels of resistance, this use is no longer recommended routinely.
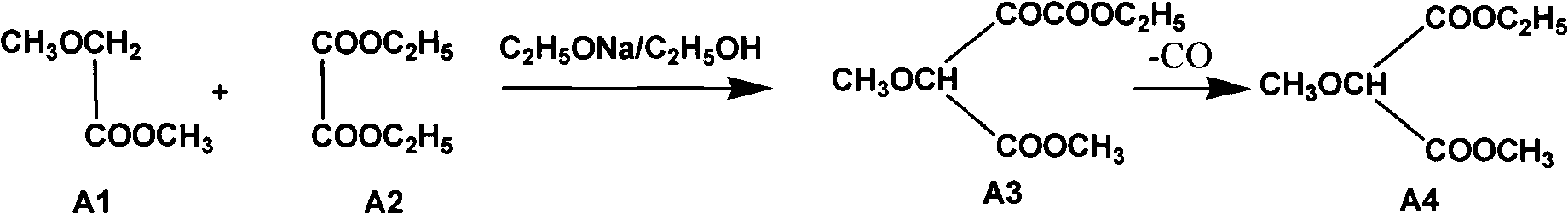
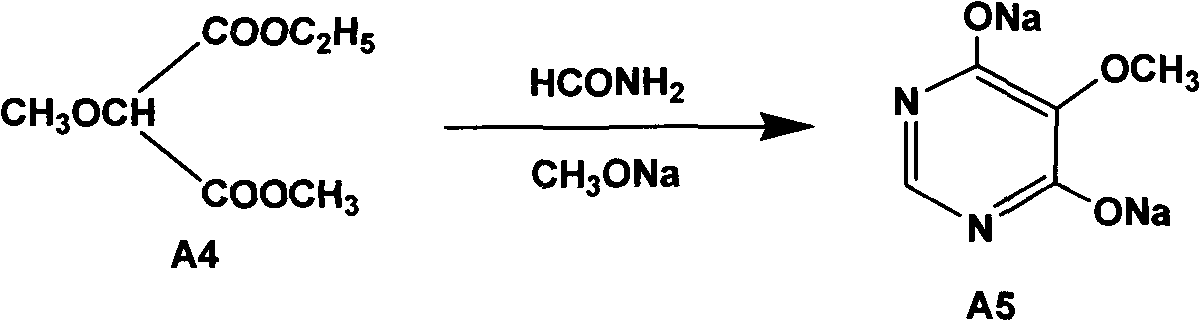
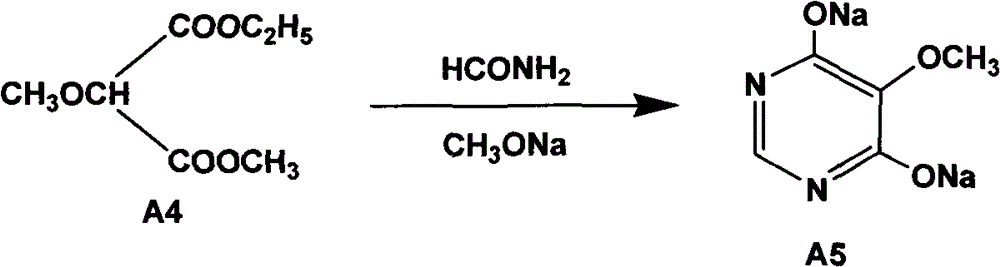
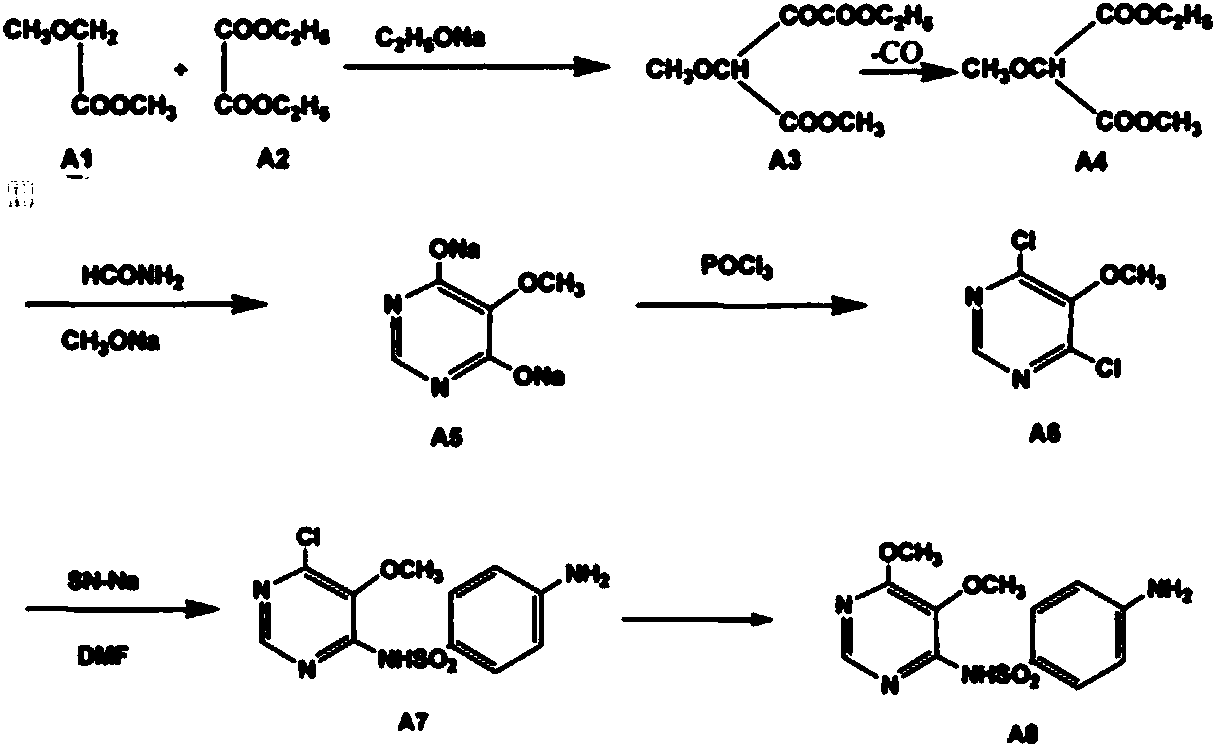
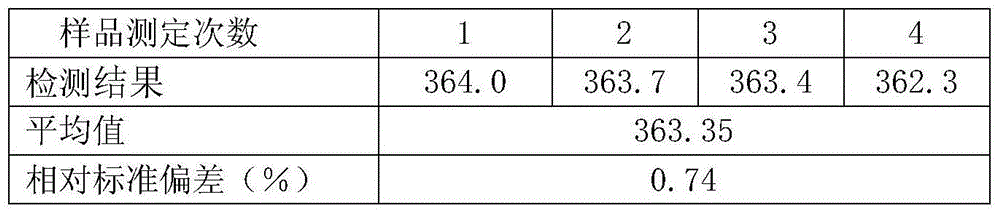
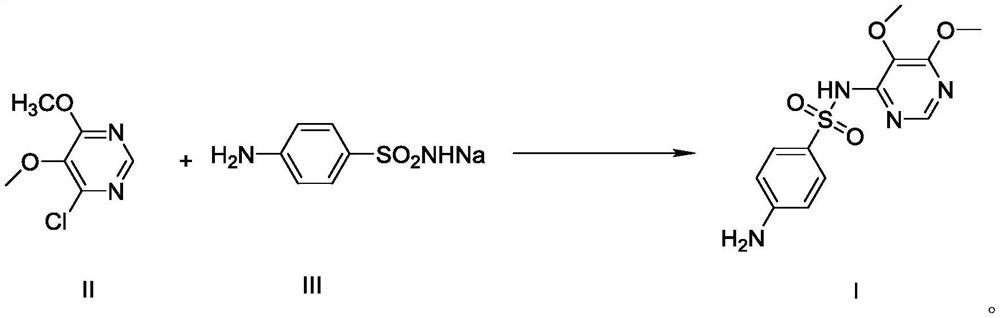
Preparation method of sulfadoxine and intermediate thereof
InactiveCN102304094AReduce manufacturing costHigh purityOrganic chemistryMethyl methoxyacetateMalonate
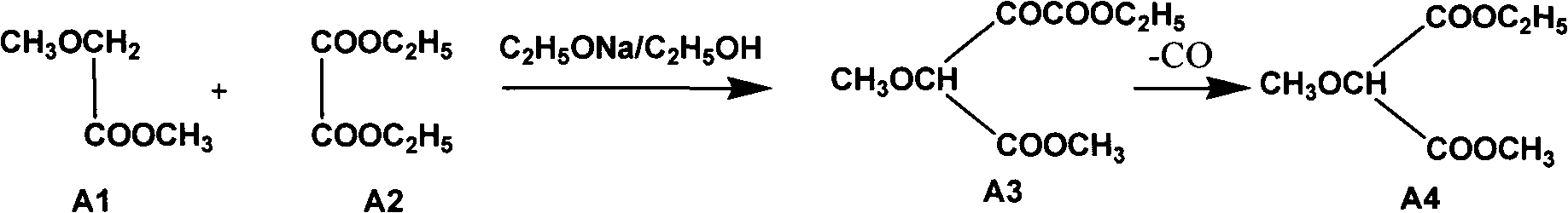
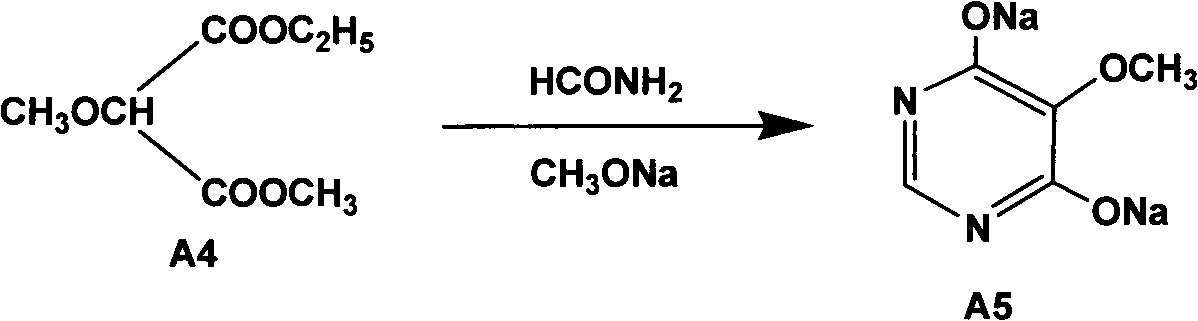
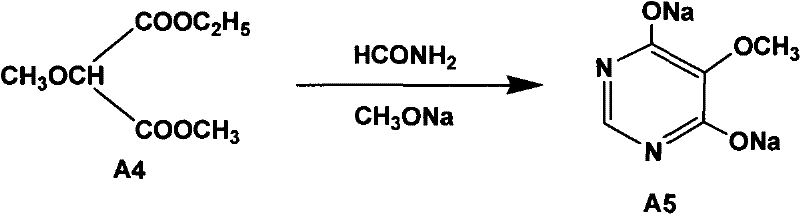
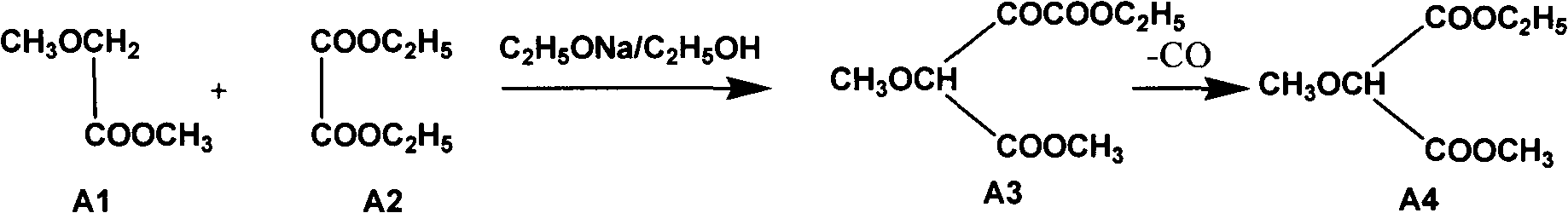
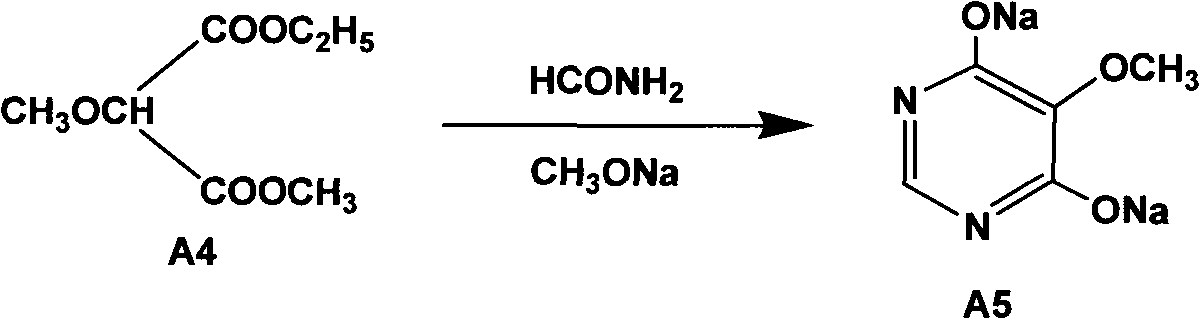
The invention relates to a preparation method of sulfadoxine and an intermediate thereof. The preparation method of sulfadoxine comprises the following steps: (1) reacting methyl methoxyacetate and excessive diethyl oxalate to generate 3-methoxy-2-oxo-methylethyl succinate, and decarbonylating to obtain 2-methoxy-methylethyl malonate; (2) reacting the 2-methoxy-methylethyl malonate and formamide to generate a cyclocompound; (3) reacting the cyclocompound and phosphorus oxychloride to generate chloride; (4) carrying out condensation reaction; and (5) carrying out methoxylation reaction. The obtained cyclocompound in the step (2) is controlled to exist in the form of anhydrous hydroxy sodium salt, so that N,N-dimethylaniline is not needed as a catalyst in the step (3), thereby lowering the cost, enhancing the quality of chloride and finally enhancing the quality of sulfadoxine.
Owner:CHANGSHU NANHU INDAL CHEM
Preparation method for sulfadoxine
Disclosed is the preparation method for sulfadoxine consisting a condensation reaction and a methyl oxidation reaction, wherein the former consists of charging N,N-dimethyl formamide, sulfonamide sodium salt, chloride into dried reaction container proportionally, then charging sulfonamide sodium salt, solid caustic soda, measuring pH=10, elevating the temperature and measuring pH=10 again, then carrying out in turn heating, heat preservation, recovering, dissolving, diluting, adjusting pH, cooling down, separating, acidifying, adjusting pH, dewatering, scouring and drying.
Owner:CHANGSHU NANHU INDAL CHEM
Preparation method of sulfadoxine
A preparation method of sulfadoxine belongs to the field of sulfanilamide antimicrobial drug preparation. Cyclization reaction comprises the following steps of: firstly pouring a sodium methoxide solution into a reactive pan, then successively adding methanamide and methyl ethyl methoxymalonate, keeping warm, recovering methanol, cooling for crystallization, drying by centrifugation, discharging,and drying to obtain 5-methoxy-4,6-disodium dihydroxypyrimidine; Chlorination reaction comprises the following steps of: firstly putting phosphorus oxychloride into a reaction vessel for heating, adding 5-methoxy-4,6-disodium dihydroxypyrimidine into the reaction vessel to react, decompressing and recovering phosphorus oxychloride until the material is dry, cooling, adding trichloro ethylene withuniformly stirring, putting into a hydrolysis pan for hydrolyzation, collecting a trichloro ethylene layer after standing and delaminating, followed by a neutralization reaction, controlling pH value, washing, removing a water layer, recovering trichloro ethylene, and releasing crystals to obtain 5-methoxy-4,6-dichloropyrimidine. The preparation method provided by the invention can be used to guarantee the product purity, prolong the service life of equipment, avoid the damage to the environment and human body, reduce emission, and save energy, and accords with foreign pharmacopoeia standard requirements.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Preparation method of sulfadoxine
InactiveCN102304095AReduce usageQuality improvementOrganic chemistrySodium methoxideDimethylaniline N-oxide
The invention relates to a preparation method of sulfadoxine, which comprises the following steps: (1) reacting methyl methoxyacetate and excessive diethyl oxalate to generate 3-methoxy-2-oxo-methylethyl succinate, and decarbonylating to obtain 2-methoxy-methylethyl malonate; (2) reacting the 2-methoxy-methylethyl malonate and formamide to generate a cyclocompound; (3) carrying out chlorination reaction; (4) carrying out condensation reaction; and (5) carrying out methoxylation reaction. The purity of the 2-methoxy-methylethyl malonate obtained in the step (1) is strictly controlled, and the cyclocompound in the step (2) is controlled to exist in the form of anhydrous hydroxy sodium salt; in the step (3), no catalyst, including N,N-dimethylaniline, is used; and in the step (5), solid sodium hydroxide is substituted for sodium methoxide solution. The invention is easy to operate, has the advantage of high product quality, greatly lowers the production cost, and has obvious economic benefit and environmental benefit.
Owner:CHANGSHU NANHU INDAL CHEM
Method for preparing sulfadoxine
The invention relates to a method for preparing sulfadoxine, and belongs to the field of the preparation of sulfanilamide antibacterial medicaments. The method comprises the following processes of: performing methyl oxidation reaction and refining, wherein the process of performing the methyl oxidation reaction comprises the following step of: adding methanol solution into a reaction kettle with a stirrer, adding sodium hydroxide, stirring to perform reflux reaction, recovering methanol until the solution is dried, adding water, continuing to recover the methanol, pressing feed liquid into a decoloration pot, regulating the pH value of the feed liquid, adding the water and a decolorizer into the feed liquid, keeping the temperature and decolorizing; and after decolorizing, performing filter pressing on the feed liquid by a filter press to a precipitation pot for precipitating, regulating the pH value of the feed liquid again, centrifuging, dehydrating, spin-drying and discharging to obtain a sulfadoxine crude product. The process of refining comprises the following steps of: adding the water into a dissolution pot, adding the sulfadoxine crude product with heating and stirring, adding calcium to obtain liquid to be decolorated, decolorating, performing the filter pressing on the decolorated liquid by the filter press to a crystallization pot, regulating the pH value, centrifuging, dehydrating to obtain a filter cake, centrifuging, spin-drying, discharging and drying to obtain a sulfadoxine finished product. The method has the advantages of high yield, stable quality of the finished product and high purity, and residues of a harmful solvent carried in the previous reaction process are reduced.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Preparation method of sulfadoxine
InactiveCN102304095BHigh melting pointReduce usageOrganic chemistrySodium methoxideDimethylaniline N-oxide
Owner:CHANGSHU NANHU INDAL CHEM
Preparation method of sulfadoxine
InactiveCN102391189AQuality improvementHigh yieldOrganic chemistryMaterials preparationReaction step
The invention relates to a preparation method of sulfadoxine, which comprises a step of carrying out meth-oxidation reaction on 4-(sulfanilamid)-5-methoxyl-6-chloropyrimidine to obtain 4-(sulfanilamid)-5,6-dimethoxy pyrimidine, and the step is implemented as follows: adding the 4-(sulfanilamid)-5-methoxyl-6-chloropyrimidine, solid sodium hydroxide and methanol into a dry reaction kettle; in the process of material preparation, controlling the system temperature below 60 DEG C; and after the material preparation is completed, slowly raising the system temperature to 65-70 DEG C, reacting under the condition of heat preservation for more than 0.5 hour, and then ending the reaction. In the final methyl oxidation reaction step in the method disclosed by the invention, solid sodium hydroxide and methanol are added to replace the existing sodium methylate, therefore, the quality and yield of the crude product are improved, the yield and inherent quality of a finished product are also obviously improved, and after refining of the crude product, a sulfadoxine product of which the melting point and appearance are obviously improved can be obtained.
Owner:CHANGSHU NANHU INDAL CHEM
Method for simultaneously screening multiples categories of drug residues in fish by using ultra performance liquid chromatography-quadrupole rod time-of-flight mass spectrometry
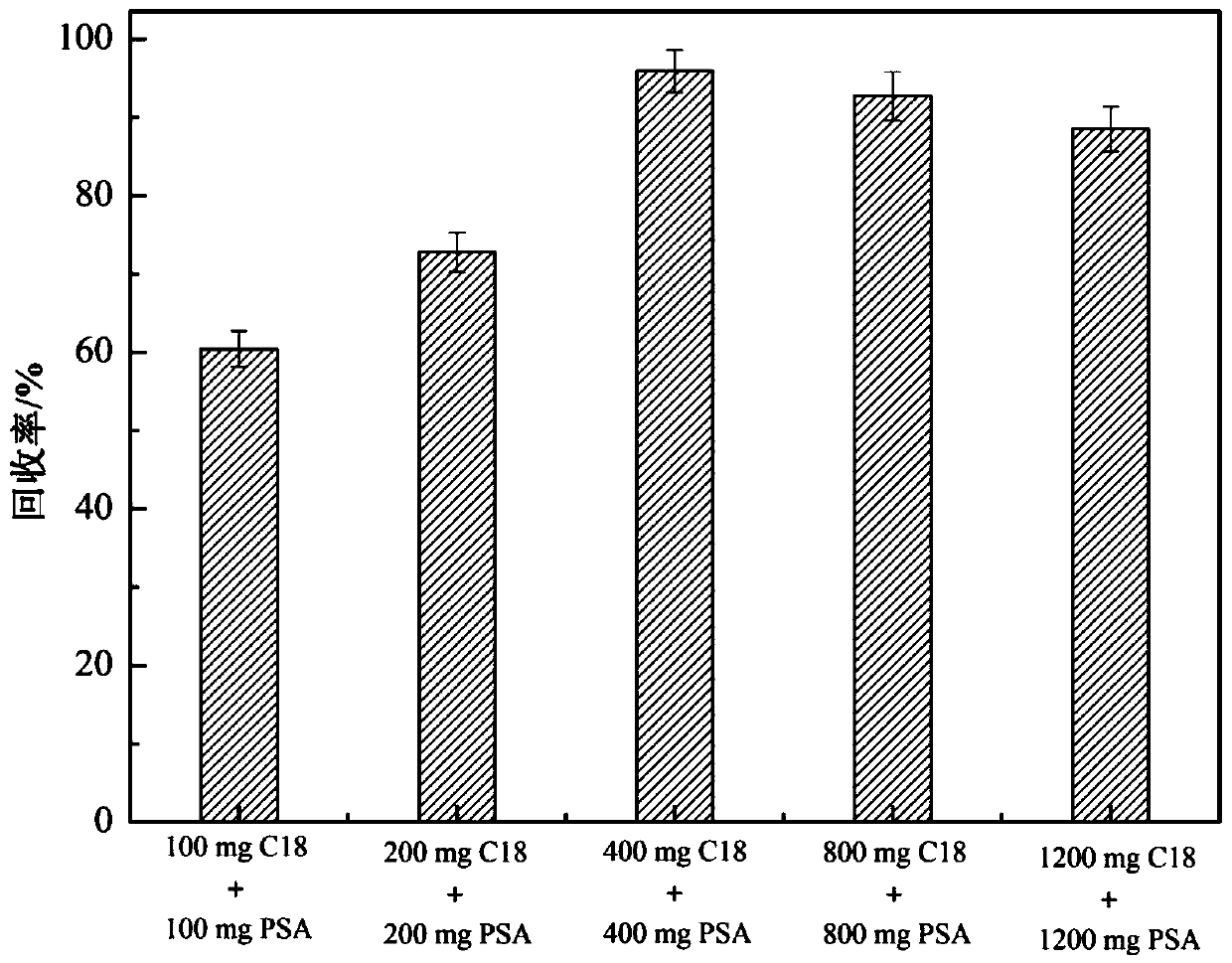
InactiveCN109917047AHigh recovery rateImprove solubilityComponent separationPretreatment methodSudan III
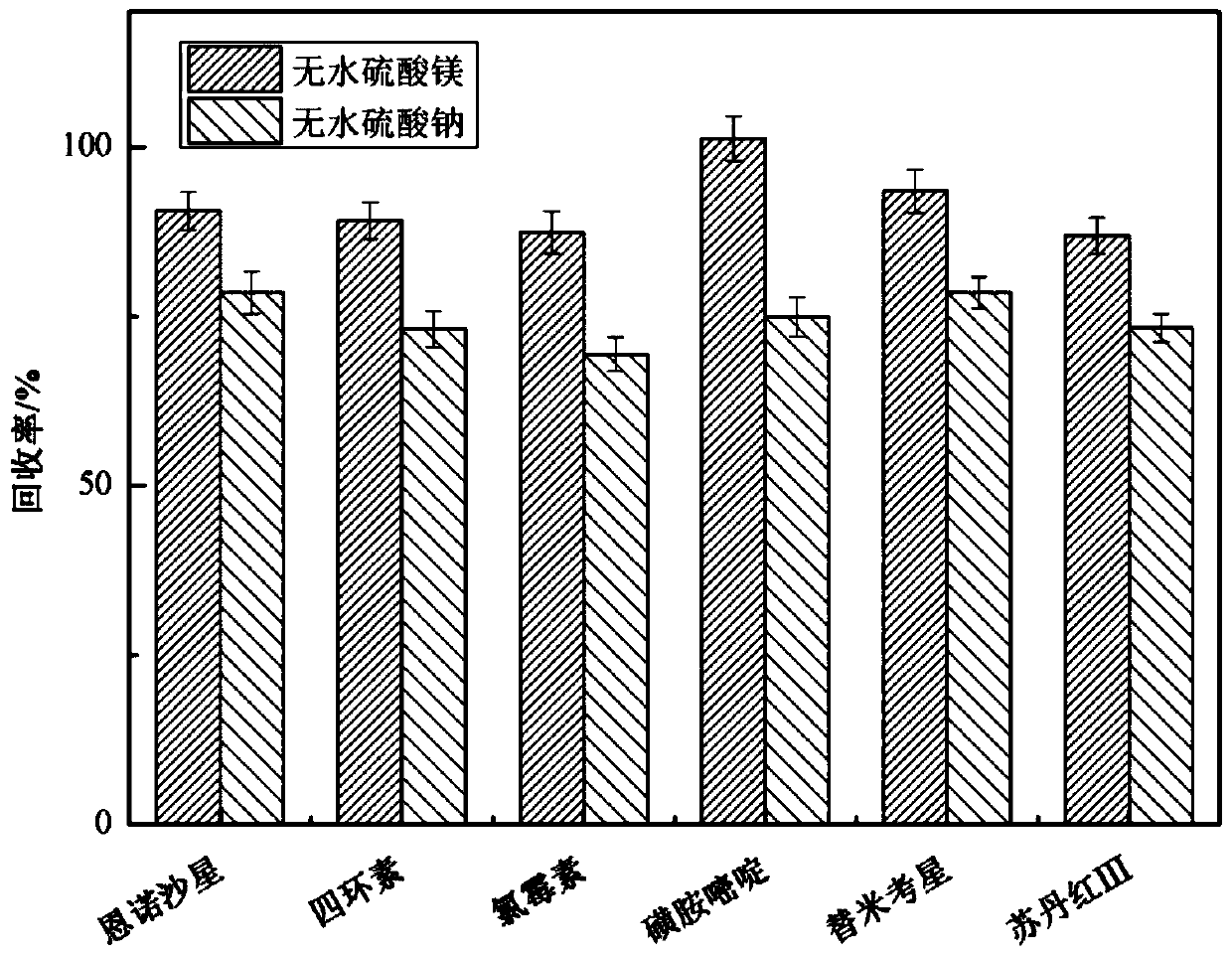
The invention relates to a method for simultaneously screening multiples categories of drug residues in fish by using ultra performance liquid chromatography-quadrupole rod time-of-flight mass spectrometry. The multiples categories of drugs screened simultaneously comprise enrofloxacin, danofloxacin, tetracycline, oxytetracycline , chlortetracycline, doxycycline, chloramphenicol, thiamphenicol, florfenicol, sulfamethoxazole, sulfamethoxazole, sulfathiazole, sulfadiazine, sulfadoxine, sulfisoxazole, sulfaphenirazole, sulfacetamide, sulfamethazine, azithromycin, tilmicosin, medimycin, roxithromycin, acetylspiramycin, doramectin, sudan I, sudan II, sudan III, sudan IV and rhodamine B. By optimizing the parameters of a d-SPE pretreatment method, all target drugs can obtain good recovery rates.29 kinds of target drugs are simultaneously screened by an optimized chromatographic mass spectrometry condition, thereby achieving simultaneous screening of common multiples categories of drug residues in fish. The method has a good application prospect in the field of fish food.
Owner:SHAANXI UNIV OF SCI & TECH
Methods for preparing sulfadoxine and intermediate of sulfadoxine
InactiveCN102432550AQuality improvementImprove qualityOrganic compound preparationCarboxylic acid esters preparationMethyl methoxyacetateDiethyl oxalate
The invention relates to methods for preparing sulfadoxine and an intermediate of the sulfadoxine. The method for preparing the sulfadoxine sequentially comprises the following steps of: (1), in the presence of sodium ethylate, reacting methyl methoxyacetate with excessive diethyl oxalate to generate 3-methoxyl-2-oxo-ethyl methyl succinate, decarbonylating the 3-methoxyl-2-oxo-ethyl methyl succinate to obtain 2-methoxyl-ethyl methyl malonate; (2) reacting the 2-methoxyl-ethyl methyl malonate with formamide to generate a cyclized compound; (3) reacting the cyclized compound with phosphorus oxychloride to generate chloride; (4) performing condensation reaction; and (5) performing methyl oxidation reaction, wherein the purity of the 2-methoxyl-ethyl methyl malonate which is obtained in the step (1) is controlled to be more than or equal to 95 weight percent. By adoption of the methods, the purity of the 2-methoxyl-ethyl methyl malonate is effectively controlled to be more than 95 weight percent, the quality of the cyclized compound for cyclization reaction in next step can be improved, operation is simplified, and production cost is reduced.
Owner:CHANGSHU NANHU INDAL CHEM
Preparation method of high-purity sulfadoxine
ActiveCN104557735AHighlight substantiveSignificant progressOrganic chemistryPhysical chemistrySulfanilamide
The invention discloses a preparation method of high-purity sulfadoxine, which comprises the following steps: adding a sulfadoxine intermediate condensate crude product into an N,N-dimethylformamide / low-polarity solvent mixed solvent, dissolving by heating, cooling to crystallize to obtain a high-purity sulfadoxine intermediate condensate, carrying out methoxylation reaction to obtain a sulfadoxine crude product, and carrying out conventional acid and alkali refinement on the sulfadoxine crude product to obtain the high-purity sulfadoxine. The method overcomes the defects in the prior art. The method has the following advantages: the HPLC (high performance liquid chromatography) total impurity content of the sulfadoxine is less than 0.4%, and the HPLC maximal single impurity content is less than 0.1%. The whole technique is simple to operate, can obtain the high-quality product, and is suitable for industrialized mass production.
Owner:CHONGQING KANGLE PHARMA
Preparation method for sulfadoxine
InactiveCN1272327CReduce heavy metal contentLow loss on dryingAntibacterial agentsOrganic chemistryChlorideCooling down
Disclosed is the preparation method for sulfadoxine consisting a condensation reaction and a methyl oxidation reaction, wherein the former consists of charging N,N-dimethyl formamide, sulfonamide sodium salt, chloride into dried reaction container proportionally, then charging sulfonamide sodium salt, solid caustic soda, measuring pH=10, elevating the temperature and measuring pH=10 again, then carrying out in turn heating, heat preservation, recovering, dissolving, diluting, adjusting pH, cooling down, separating, acidifying, adjusting pH, dewatering, scouring and drying.
Owner:CHANGSHU NANHU INDAL CHEM
Purification method of sulfadoxine
The invention discloses a purification method of sulfadoxine. The purification method comprises the following steps: 1) adding 13.4 parts by mass of deionized water to a reaction container, next, adding 2.4 parts by mass of calcium hydroxide, increasing the temperature to 75 DEG C, slowly adding 5.3 parts by mass of sulfadoxine crude product, then adding 1.3 parts by mass of calcium hydroxide, regulating the PH value to 8.4, and stirring evenly; 2) increasing the temperature to 90 DEG C, then adding activated carbon, naturally cooling and filtering to obtain the filtrate; 3) increasing the filtrate to 65 DEG C, dropwise adding 45% of glacial acetic acid solution, regulating the PH value to 4.8, standing for precipitating and then centrifugally dehydrating to obtain the filter residue; and 3) drying the filter residue at 145 DEG C for 1 hour, cooling to 25 DEG C and then discharging the material. The purification method is capable of improving the purity and yield of the sulfadoxine.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Quality control material for metabolomics detection and quality control method of quality control material
The invention discloses a quality control material for metabolomics detection and a quality control method of the quality control material. The quality control material comprises mixed standard samples of 4,4'-methylene bis(2-chloroaniline), p-anisidine, L-tyrosine methyl ester, 3-chloroaniline, 2,4-dimethyl quinoline, sulfapyridine, atrazine, sulfadoxine, DL-leucine, N-benzoyl-L-tyrosine ethyl ester, 6-phenyl-2-thiouracil, N-(o-toluoyl) glycine, 2-methyl-5-nitroimidazole-1-ethanol, glycyrrhetinic acid, flavanone, Epsilon-caprolactone and 2-aminopyridine. 17 standard samples in the method comefrom different material categories and are very stable, simple in preparation and small in amount; and the quality control material disclosed by the invention can accurately reflect the state of a chromatograph or a mass spectrometer.
Owner:嘉兴迈维代谢生物科技有限公司
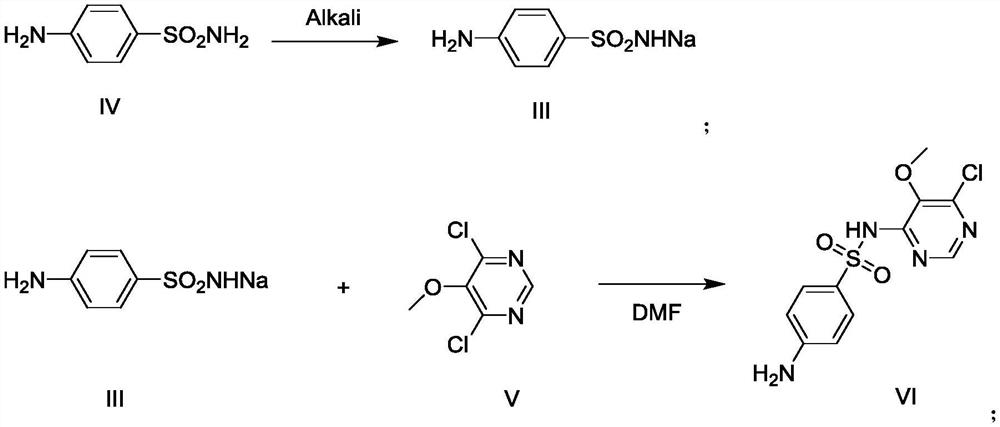
Preparation method for preparing sulfonamide sodium salt of sulfadoxine
InactiveCN102584648AReduce consumptionReduce bring inSulfonic acid amide preparationPhysical chemistryPharmaceutical drug
The invention discloses a preparation method for preparing a sulfonamide sodium salt of sulfadoxine, belonging to the technical field of preparation of a sulfonamide antibacterial medicament. The method comprises the following steps of: putting solid sodium hydrate, technical grade sulfonamide or recrystallized sulfonamide and water into a reaction pot with a stirrer; heating and dissolving in a stirring state; after the solid sodium hydrate and the recrystallized sulfonamide are fully dissolved, continually heating and carying out distilling for removing water to dryness; further heating till materials in the reaction pot become powder; and discharging and drying to obtain the sulfonamide sodium salt of which the sodium content is more than or equal to 90 percent and the nitrogen content is more than or equal to 92 percent. The method has the advantages that: the sodium content and the nitrogen content of the obtained sulfonamide sodium salt are stable; the quality of the sulfonamide sodium salt can be guaranteed, the reaction efficiency of a next procedure is ensured, and impurities in a finished product are reduced; impurities brought into the finished product can be reduced, and the product purity is ensured; and a final finished product, i.e. sulfadoxine, prepared from the sulfonamide sodium salt obtained by the method has high purity.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
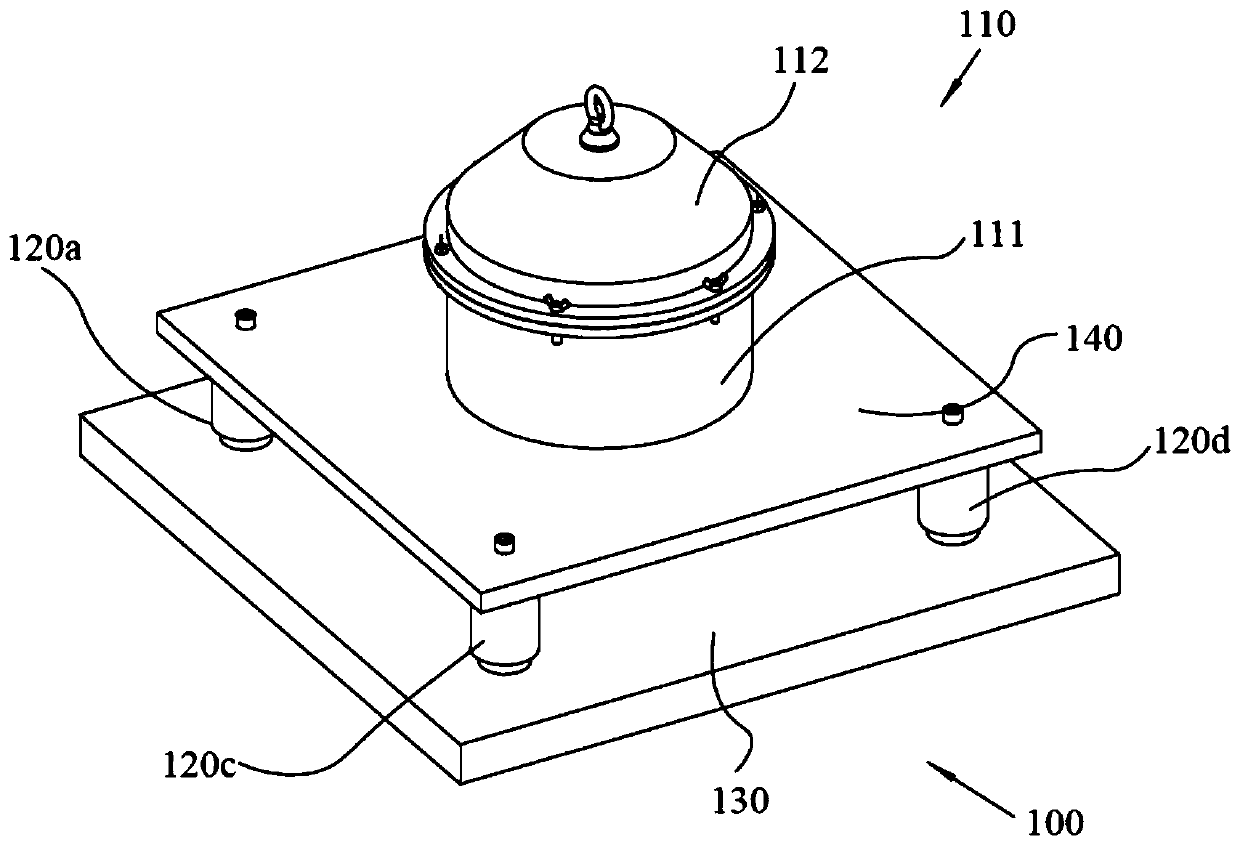
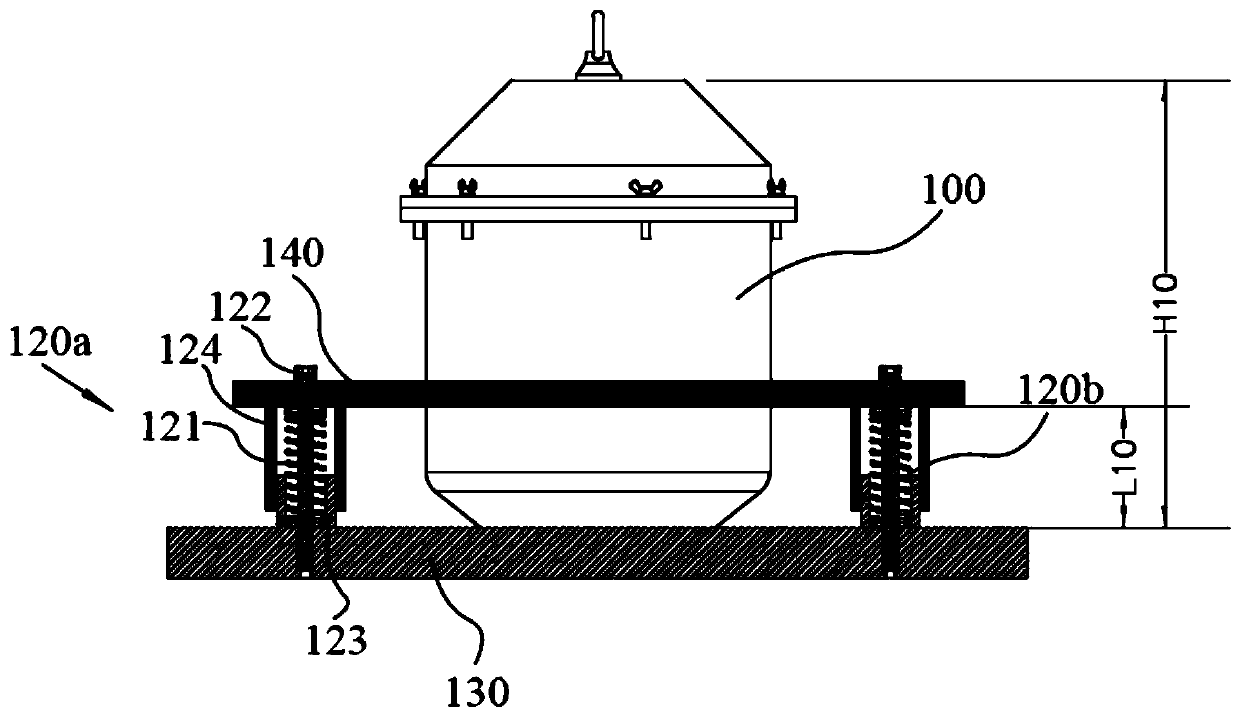
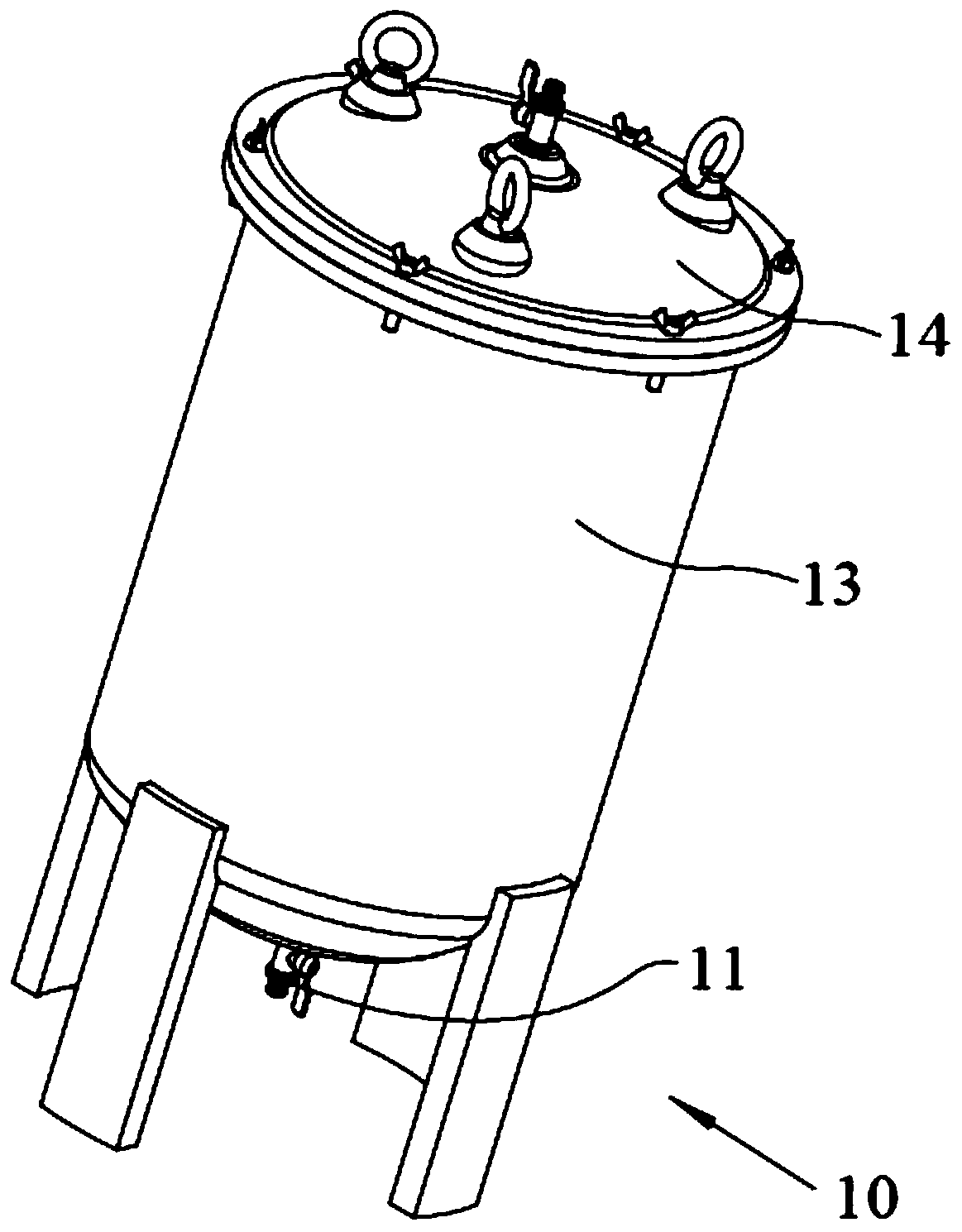
Efficient and safe centrifugal process for sulfadoxine
InactiveCN110762162AGuaranteed uptimeAvoid the problem of increased eccentric vibrationCentrifugesVibration suppression adjustmentsSulfanilamideStructural engineering
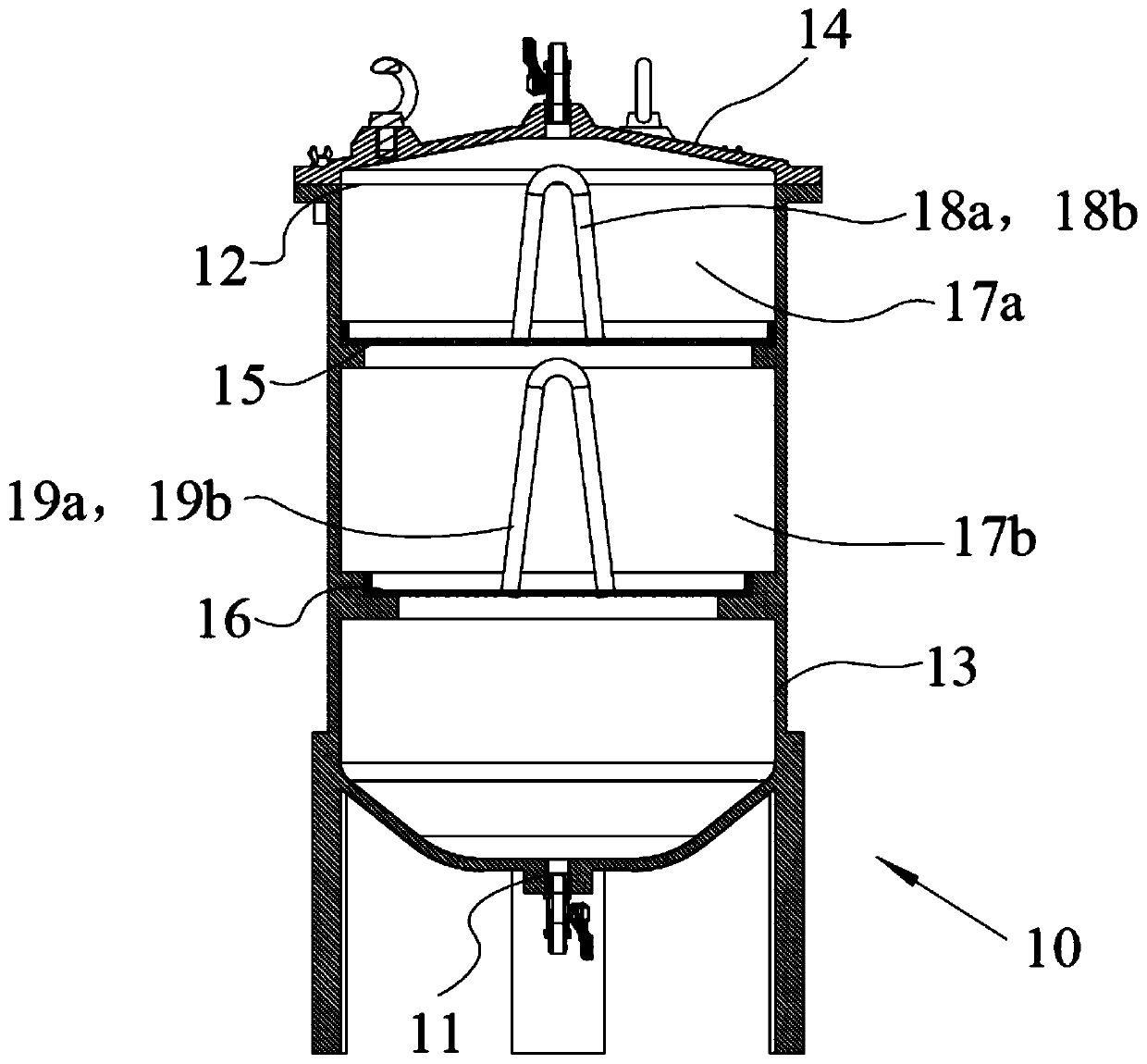
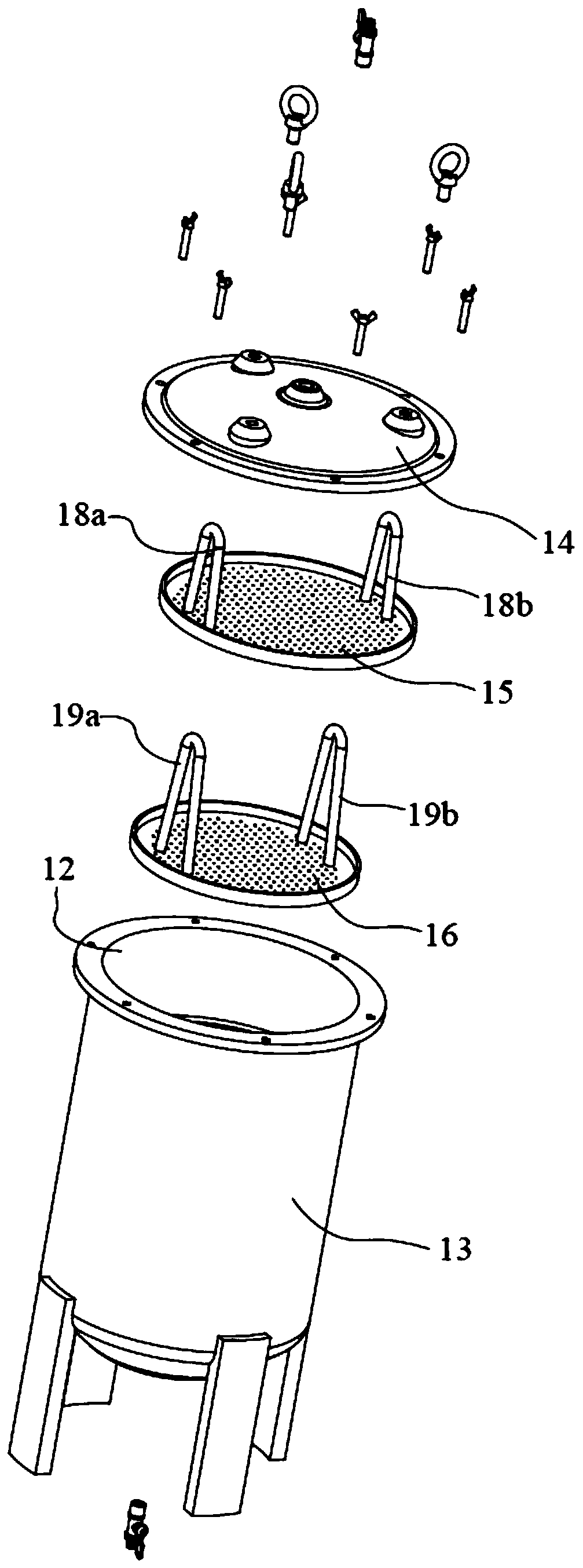
The invention discloses an efficient and safe centrifugal process for sulfadoxine. Centrifugal procedures are finished in an efficient and safe centrifugal separation device; the efficient and safe centrifugal device comprises a filter centrifuge main body; the filter centrifuge main body is mounted on a fixed base through spring sleeving installation modules with an eccentric adjusting function at the periphery; each spring sleeving installation module comprises an eccentric adjusting spring, a fixed locking screw rod, an embedding base pipe integrally arranged on the fixed base, and a mounting sleeve fixedly arranged on an upper mounting plate; the mounting sleeves sleeve the outer peripheries of the embedding sleeves; the eccentric adjusting springs are arranged between the upper mounting plates and the fixed base, and are positioned at the inner peripheries of the embedding sleeves; and fixed locking screw rods are inserted in the eccentric adjusting springs for threaded locking fit with the internal of the fixed base. The efficient and safe completion of sulfadoxine centrifugal procedures in the preparation process can be reliably guaranteed; the long-time reliable operation of the centrifugal procedures is guaranteed; and meanwhile, potential safety hazards are prevented.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
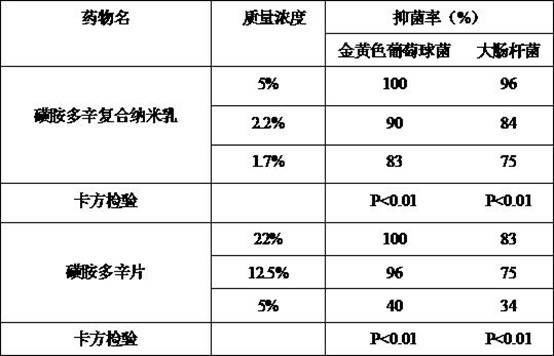
Sulfadoxine composite nanoemulsion antibacterial medicine and preparation method thereof
InactiveCN102657609AImprove bioavailabilityExtended half-lifeAntibacterial agentsHydroxy compound active ingredientsBiotechnologyAntimicrobial drug
The invention discloses a sulfadoxine composite nanoemulsion antibacterial medicine. The sulfadoxine composite nanoemulsion antibacterial medicine comprises the following components in percentage by mass: 0.01 to 1.4 percent of sulfadoxine, 3 to 5.8 percent of oil, 28 to 35 percent of surfactant, 0 to 8.4 percent of cosurfactant and the balance of distilled water, wherein the mass percentage sum of the components is 100 percent. The sulfadoxine composite nanoemulsion antibacterial medicine is a clear and transparent liquid, has the characteristics of low viscosity, high stability, high dispersibility, quickness in absorption and the like, can improve the bioavailability of the medicine, prolongs the half-life period of the medicine in vivo, enhancea the curative effect, reduces the toxicity, has sustained release and targeted effects, and has the advantages of low energy consumption during manufacturing, low toxicity, high safety and the like.
Owner:NORTHWEST A & F UNIV
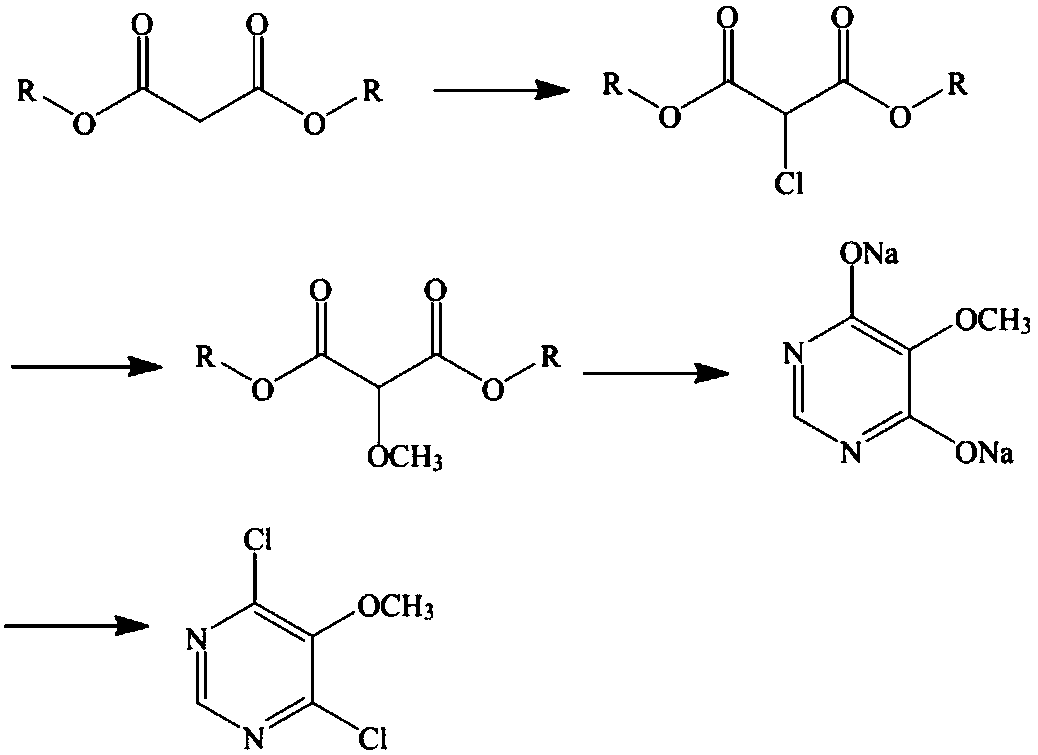
Preparation method of sulfadoxine midbody 4,6-dichloro-5-methoxypyrimidine
ActiveCN108658871AReduced purification stepsSave time and costOrganic chemistryMalonic acidContinuous flow
The invention belongs to the technical field of the preparation of a sulfadoxine midbody, and particularly relates to a preparation method of the sulfadoxine midbody 4,6-dichloro-5-methoxypyrimidine.The method comprises the following steps: (1) adopting malonic acid diester as a raw material, and successively performing the chlorination to prepare 2-chloro-malonic acid diester; (2) performing themethoxidation reaction, and obtaining 2-methoxyl-malonic acid diester; (3) performing the cyclization reaction, and obtaining 4,6-dihydroxyl-5-methoxypyrimidine disodium salt; and (4) performing thesecondary chlorination, and obtaining 4,6-dichloro-5-methoxypyridine. By adopting the preparation method, the purification steps can be reduced, the continuous flow and no material discharge of the production process can be realized, and the continuous flow process is environment-friendly and safe; and moreover, at least two hours can be saved, the time cost can be reduced, and the industrial production efficiency can be improved.
Owner:舞阳威森生物医药有限公司
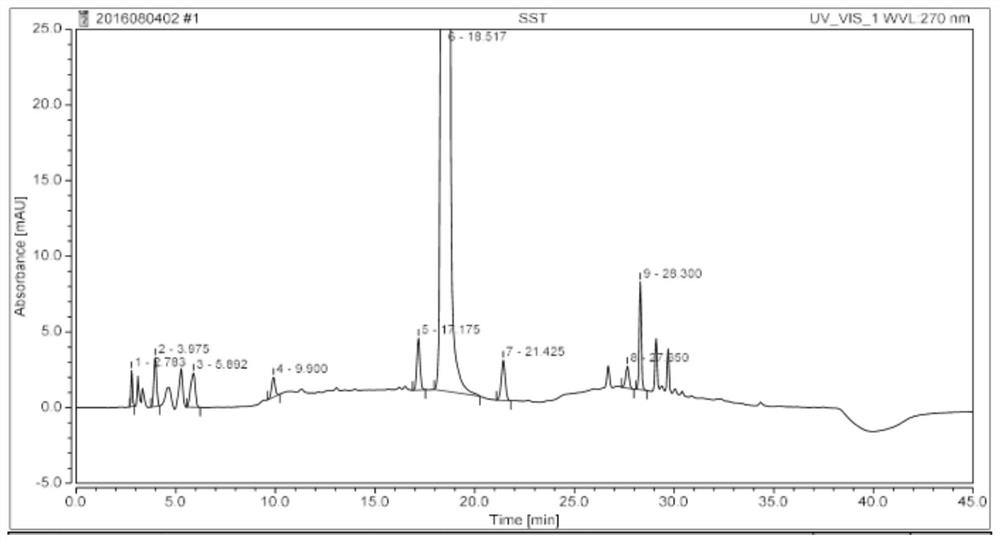
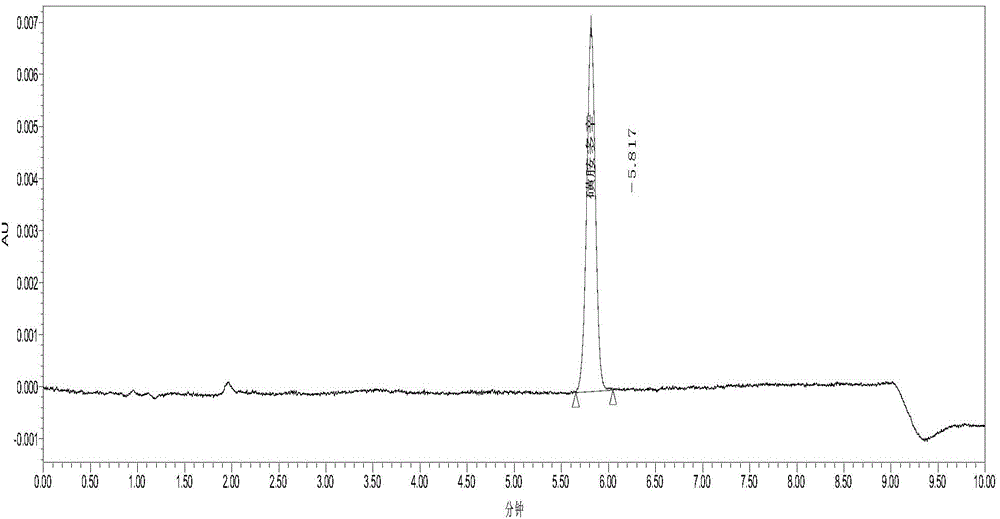
Method for analyzing residual quantities of residual solvents in sulfadoxine
The invention discloses a method for analyzing the residual quantities of residual solvents in sulfadoxine. The method comprises the following steps: (1) preparing an internal standard solution; (2) preparing a contrast stock solution; (3) preparing a contrast solution; (4) a preparing a test solution; (5) precisely measuring 5 microliters of the contrast solution, injecting the contrast solution into a gas chromatograph, and recording a chromatogram; (6) precisely measuring 5 microliters of the test solution, injecting the test solution into the gas chromatograph, and recording the chromatogram; and (7) calculating the residual quantity of each solvent in a sample according to an internal standard method according to a formula that the residual quantity of each solvent in the sample is equal to the quotient of peak areas of the residual solvent and an internal standard solvent. The method, provided by the invention, can be used for analyzing the residual quantities of various residual solvents in the sulfadoxine.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
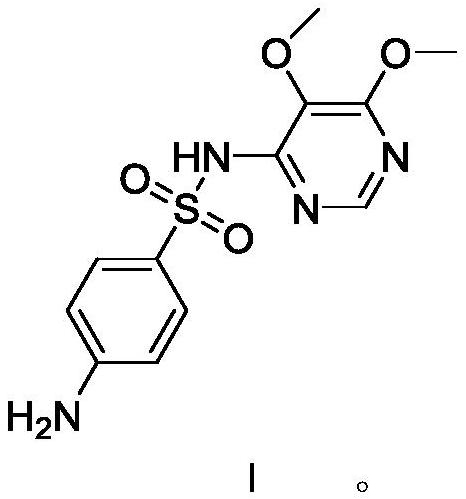
Preparation method of sulfadoxine derivative
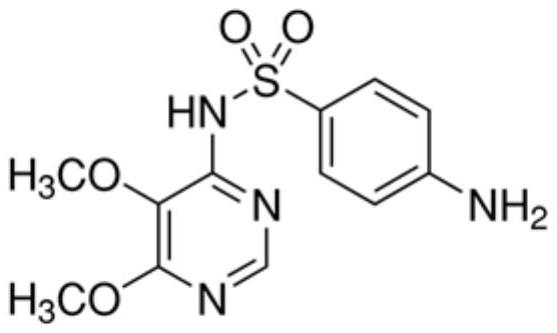
The invention discloses a preparation method of a sulfadoxine derivative I, namely, 4-(4-acetaminobenzenesulfonamido)-5,6-dimethoxy pyrimidine. The sulfadoxine derivative I is an acetylized impurity of sulfadoxine and is one of main impurities of a sulfadoxine active ingredient or preparation; and the sulfadoxine derivative I can be used for analyzing and detecting the purity of the sulfadoxine, thereby controlling the quality of the sulfadoxine.
Owner:CHONGQING KANGLE PHARMA
Method for one-pot synthesis of sulfadoxine by monitoring reaction progress through HPLC
Owner:GUILIN PHARMA
Finishing treatment method of sulfadoxine
The invention discloses a finishing treatment method of sulfadoxine. According to the method, finishing is performed on a crude sulfadimethoxine product generated through a cyclization reaction and achlorination reaction, and an alkaline aqueous solution with a pH value in a range of 8-12.5 is used as a solvent; the crude sulfadimethoxine product is completely dissolved in the alkaline aqueous solution to obtain an alkaline aqueous solution of the crude sulfadimethoxine product, wherein the alkaline aqueous solution of the crude sulfadimethoxine product comprises an impurity A and an impurityB; activated carbon comprises non-pre-activated activated carbon and acidic pre-activated pore-expanded activated carbon, and the acidic pre-activated pore-expanded activated carbon is prepared by soaking activated carbon in an acetic acid or hydrochloric acid solution in advance, carrying out boiling for 2-4 hours and then performing drying before addition into the alkaline aqueous solution of the crude sulfadimethoxine product; and the activated carbon and the acidic pore-expanded activated carbon are added into the alkaline aqueous solution of the crude sulfadimethoxine product at the sametime to decolorize and adsorb impurities in the alkaline aqueous solution of the crude sulfadimethoxine product. According to the invention, a decolorization process is specially designed and improved, so the finishing purity of sulfadoxine can be obviously improved; and the adopted finishing treatment method is simple to operate.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Sulfadoxine production process
The invention discloses a sulfadoxine production process. The sulfadoxine production process includes the steps: 1) enabling reaction of methyl methoxyacetate and diethyl oxalate to generate 3-methoxy-2-ethyl oxo-succinate formate; 2) in existence of sodium methoxide, enabling 2-methoxy-ethyl malonate formate to react with formamide, adding water, recovering aqueous methanol, spinning, discharging and drying to obtain a ring compound; 3) enabling the ring compound and phosphoryl chloride to flow back at 65 DEG C, and performing heat reaction for 3 hours to generate 4,6-dichloro-5-methoxy pyrimidine; 4) subjecting the 4,6-dichloro-5-methoxy pyrimidine and aminophenol sulfamide sodium to condensation reaction to generate 4-(sulfanilamide)-5-methoxy-6-chloropyrimidine; 5) putting the 4-(sulfanilamide)-5-methoxy-6-chloropyrimidin into a reaction kettle, adding methanol and solid sodium hydroxide, slowly heating to 60 DEG C, and performing heat reaction for 2.5 hours to obtain a finished sulfadoxine product. The sulfadoxine production process has the advantages of high sulfadoxine production yield.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
In-vitro diagnostic reagent preservative and application thereof
PendingCN113897413AGood antibacterial effectImprove stabilityMicrobiological testing/measurementBiological material analysisTrimethoprimMedicine
The embodiment of the invention belongs to the technical field of medical examination and determination, and relates to an in-vitro diagnostic reagent preservative. The in-vitro diagnostic reagent preservative comprises a composition of a sulfadoxine solution and a diaveridine solution, and the molar ratio of sulfadoxine in the sulfadoxine solution to diaveridine in the diaveridine solution is 0.002 to 1. The invention further relates to application of the in-vitro diagnostic reagent preservative. According to the technical scheme, the stability of the reagent can be improved, reagent reaction is not affected, and the reagent can be widely used.
Owner:DAAN GENE CO LTD
Composition containing sulfadoxine
InactiveCN103191438AImprove stabilityGuaranteed reliabilityOrganic active ingredientsAntiparasitic agentsGlycerolDodecylsulfonic acid
The invention relates to a composition containing sulfadoxine. The composition also contains the following components: 10-20wt% of deoxysodium cholate, 30-40wt% of glycerin, 20-50wt% of L-glutathione and 20-30wt% of sodium dodecyl sulfate. The composition has the beneficial effect of good stability.
Owner:天津市嘉凡生物科技有限公司
Sulfadoxine preparation process
The invention discloses a sulfadoxine preparation process which comprises the following specific preparation process steps: 1, technicists are familiar with a special scheme of the sulfadoxine preparation process in detail, put forward doubts and solve the doubts, and finally submit a consistent special scheme to a technical director for approval; 2, the methanol solution is added into a reactionkettle with a stirrer, sodium hydroxide is added, stirring is started, 4-(p-aminobenzenesulfonamido)-5-methoxy-6-chloropyrimidine is added, a reflux reaction is carried out, the reflux reaction temperature and the reflux reaction time are controlled, and methanol is recovered to dryness after the reaction ends in order to obtain sodium methoxide; 3, 184 g of 27%-28% sodium methoxide is put into the reaction bottle, methyl alcohol is evaporated to dryness, then a mixed solution of 83 g of methyl methoxyacetate and 142 g of diethyl oxalate is put into the reaction bottle, and the temperature rises naturally. The sulfadoxine preparation process is stable, reliable, simple in equipment, low in manufacturing cost, convenient to operate, high in practicability and suitable for wide application and popularization.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
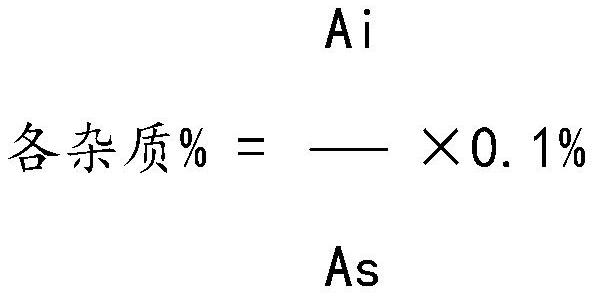
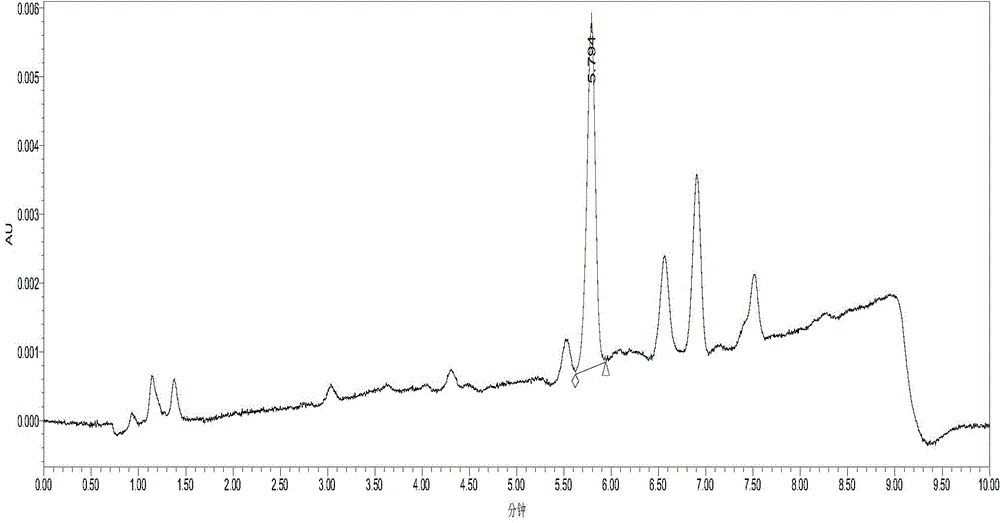
Method for analyzing related substances of sulfadoxine
The invention discloses a sulfadoxine related substance analysis method which comprises the following steps: 1) preparing an applicable solution: taking about 10mg of sulfanilamide sodium, about 10mg of chloride, about 10mg of condensation compound, about 10mg of impurity A, about 10mg of impurity B, about 10mg of impurity C, about 10mg of 4-amino-5, 6-dimethoxypyrimidine and about 10mg of sulfanilic acid, precisely weighing, respectively putting into 10ml measuring flasks, firstly adding 4ml of acetonitrile, then dissolving and diluting with a mobile phase to a scale, uniformly shaking, and measuring the content of sulfanilamide sodium, chloride, condensation compound, impurity A, impurity B, impurity C, 4-amino-5, 6-dimethoxypyrimidine and sulfanilic acid; taking as an impurity stock solution A; respectively and precisely measuring 1.0 ml of the impurity stock solution A into a 100ml measuring flask, diluting to a scale by using a mobile phase, and uniformly shaking to obtain an impurity stock solution B; and precisely measuring 2.5 ml of the impurity stock solution B, adding the impurity stock solution B into a 50ml measuring flask, precisely weighing about 25mg of sulfadoxine, adding the sulfadoxine into the 50ml measuring flask, adding 20ml of acetonitrile, carrying out ultrasonic treatment to dissolve the sulfadoxine, diluting with a mobile phase to a scale, and uniformly shaking to obtain the sulfadoxine-containing organic impurity detection reagent.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
High-purity decoloration filter pressing process of sulfadoxine
ActiveCN110694319ADecolorization ensuresHigh yieldOrganic chemistryMembrane filtersActivated carbonSulfanilamide
The invention discloses a high-purity decoloration filter pressing process of sulfadoxine. According to the process, an active carbon decolorization process comprises: adding active carbon into a crude sulfadoxine dissolving solution to adsorb impurities, cooling, feeding into a filter press, and filtering; the filter press comprises a filter pressing cavity, wherein the bottom of the filter pressing cavity is provided with a sulfanilamide filtrate outlet, and the upper portion of the filter pressing cavity is provided with a feeding port; a filter pressing cavity cover plate is cooperativelymounted at the feeding port; the filter pressing cavity is connected to an external pressure device through the filter pressing cavity cover plate, and is used for injecting pressure into the filter pressing cavity; a first filter pressing mechanism and a second filter pressing mechanism are vertically distributed at intervals and are fixedly arranged on the inner periphery of the filter pressingcavity; the first filter pressing mechanism is close to the feeding port, and has a mesh number of 60-80; and the second filter pressing mechanism is close to the sulfanilamide filtrate outlet, and has a mesh number of 100-120. According to the invention, through the structural design of the filter pressing device, the removal of the high-content decolorizing agent and the high-content impuritiesin the decoloration process can be ensured, and the purity of the sulfadoxine finished product is further ensured.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
A kind of sulfadoxine residue reference substance with turbot muscle as matrix and preparation method thereof
The invention relates to a sulfadoxine residual primary standard substance utilizing a turbot muscle as a matrix and a preparation method thereof. The preparation method comprises the specific steps of culturing the turbot the weight of which is 500-600g in a mariculture system, carrying out medicine preparation, filling and sampling the turbot. According to the sulfadoxine residual primary standard substance and the preparation method thereof provided by the invention, a sulfadoxine medicine in a turbot body can be really subjected to a digestion process, an absorbing process, a delivering process, an accumulating process, a metabolizing process and an eliminating process and other processes to be left in the muscle to prepare the standard substance, the sulfadoxine residual primary standard substance does not utilize the fish muscle as a base, and a medicine is added to prepare the standard substance, the property of the standard substance is close to the practical property of a detection sample, operations such as the evaluation of detection method, the inspection of the difference among laboratories, the verifying of the result accuracy of operators, which are performed by the substance, are scientific and exact.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Method for preparing sulfadoxine
The invention discloses a method for preparing sulfadoxine. The method comprises the following steps: 1) inputting a trichloro ethylene solution of 4-(p-amino benzenesulfonamido)-5-methoxyl-6-chloropyrimidin into a reaction container, then adding sodium hydroxide and water, stirring for 30 minutes, then adding methanol, and heating to 75 DEG C for reflux reaction for 3.5 hours to form a mixture; 2) distilling the mixture at 85 DEG C to remove methanol, raising the temperature to 120 DEG C and distilling to remove trichloro ethylene, then adding hydrochloric acid, adjusting the pH value to 9.1, and then adding active carbon to stir and decolor for 2.5 hours to obtain a clean liquid; and 3) raising the temperature of the clean liquid to 65 DEG C, titrating a 45% glacial acetic acid solution, adjusting the pH value to 4.7, precipitating and centrifugalizing and dehydrating to obtain the sulfadoxine. The method for preparing sulfadoxine disclosed by the invention can be used for effectively reducing heat emitted by reaction, so that the reaction is stably carried out.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
Preparation method of sulfadoxine
PendingCN112457259AHigh process yieldAvoid it happening againOrganic chemistrySulfanilamideProcess engineering
The invention provides a preparation method of sulfadoxine. The method is a preparation method of sulfadimethoxine, and the sulfadimethoxine product is prepared by directly taking 4-chloro-5, 6-dimethoxypyrimidine and sodium sulfanilamide as raw materials through a condensation reaction. The method is simple in process, free of special requirements on equipment, simple and convenient to operate, good in product quality and suitable for industrial production.
Owner:CHONGQING KANGLE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com