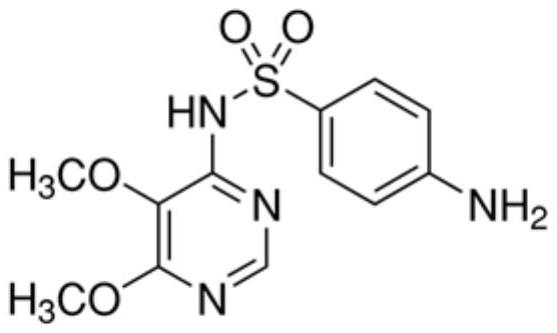
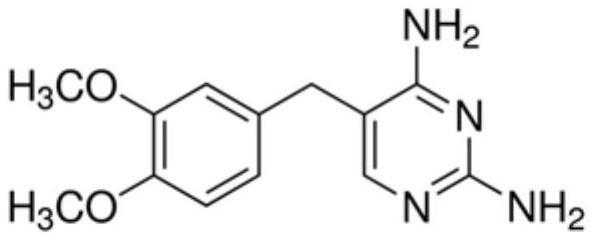
In-vitro diagnostic reagent preservative and application thereof
An in vitro diagnosis and preservative technology, which is applied in the field of medical testing and determination, can solve the problems such as the decrease in the measurement precision of diagnostic reagents, the decrease in the activity of tool enzymes and antibodies, and the toxicity of preservatives. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
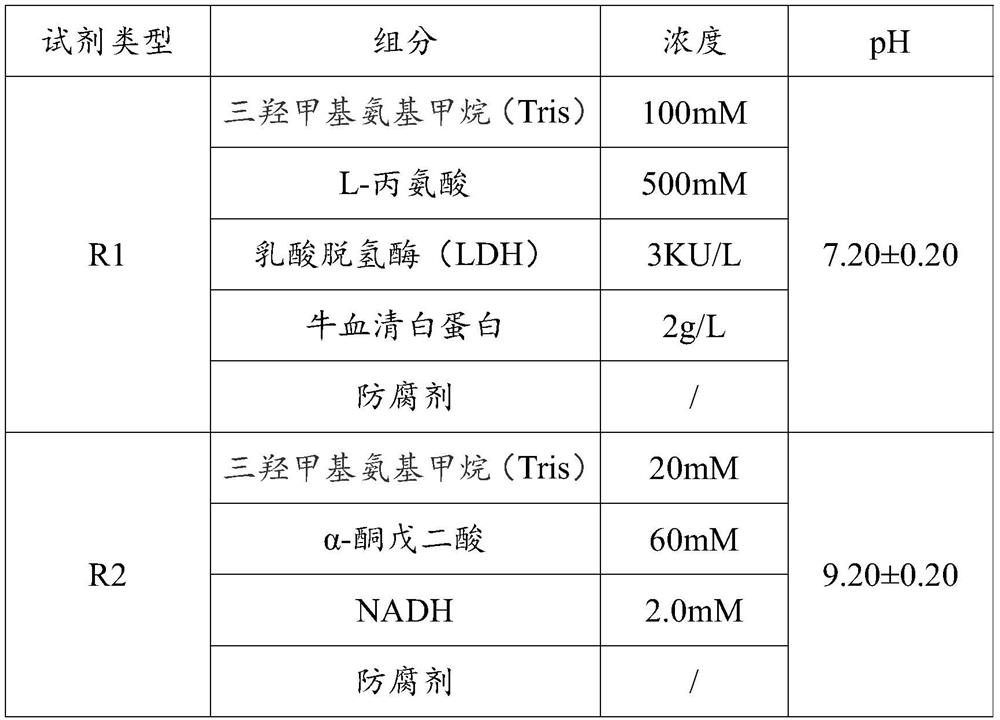
[0037] Prepare aspartate aminotransferase (AST) assay reagents according to the components and concentrations in Table 1 below.
[0038] Table 1
[0039]
[0040] In this embodiment, the in vitro diagnostic reagent is an aspartate aminotransferase (AST) assay reagent, and the AST assay reagent is a dual reagent, the dual reagents are the first type reagent R1 and the second type reagent R2, and the dual reagents are stored separately. When in use, the AST assay reagent is prepared by mixing the first type reagent R1 and the second type reagent R2.
[0041] The components of AST assay reagents include trishydroxymethylaminomethane (Tris), L-alanine, lactate dehydrogenase (LDH), bovine serum albumin, α-ketoglutarate and NADH (Nicotinamide adenine dinucleotide, also Prototype coenzyme I), the above-mentioned components were added to purified water according to the concentration in Table 1, mixed and evenly mixed, and the pH value was adjusted.
[0042] Here, the preservative...
Embodiment 2
[0050] Prepare triglyceride (TG) assay reagents according to the components and concentrations in Table 3 below.
[0051] table 3
[0052]
[0053] In this embodiment, the in vitro diagnostic reagent is a triglyceride (TG) assay reagent, and the TG assay reagent is a single reagent. The components of TG assay reagents include piperazine-1,4-diethanesulfonic acid (PIPES), magnesium chloride hexahydrate, adenosine triphosphate, p-chlorophenol, ethylenediaminetetraacetic acid, Triton TX-100, lipoprotein lipase, Glycerol phosphate oxidase, glycerol kinase and peroxidase, the above-mentioned components were added to purified water according to the concentration in Table 3, mixed and evenly mixed, and the pH value was adjusted.
[0054] Here, the preservative is added to the TG measurement reagent using the above-mentioned sulfadoxine solution and trimethoprim solution. Two groups of TG assay reagents are set as a control group, the first group of TG reagents add the compositio...
Embodiment 3
[0061] Prepare reagents according to the components and concentrations in Table 5 below, as shown in Table 5:
[0062] table 5
[0063]
[0064] In this embodiment, the reagents are set into 3 groups, and the components of each group of reagents include disodium hydrogen phosphate, potassium chloride, sodium chloride and bovine serum albumin, and the above components are respectively added to the concentration in Table 5 and mixed with purified water. Mix well and adjust the pH. Three groups of reagents were added with different preservatives, group A was without addition, group B was added with 0.5g / L sodium azide as a preservative, and group C was added with preservatives containing sulfadoxine 6 μM and ditrimethoprim 360 μM.
[0065] The 3 groups of test reagents were stored in an incubator at 37°C for 4 weeks, and finally the bacterial confirmation test was carried out on each test material after storage.
[0066] Bacteria confirmation test: Smear and scrape small sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com