Preparation method of sulfadoxine
A technology of sulfadoxine and sulfadoxine sodium, which is applied in the field of preparation of sulfadoxine, can solve problems such as complex processes and quality, and achieve the effects of simple process operation, high yield and no production equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
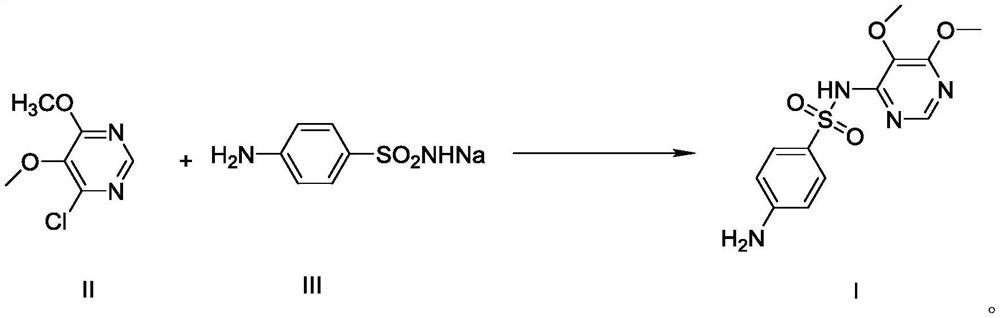
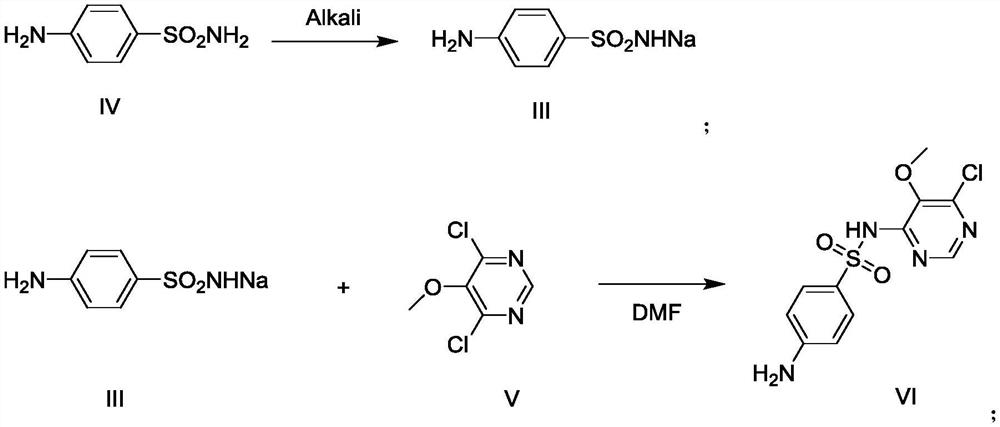
[0030] Example 1 Preparation of 4-chloro-5,6-dimethoxypyrimidine formula II
[0031] (see Bretschneider et al., Monatsh Chem. 96, 1661-1669 (1965))
[0032] Add 800g of methanol and 60g of sodium methoxide (1.11mol) into the reaction flask respectively, cool to 0°C~5°C, then add 179g (1.0mol) of 4,6-dichloro-5-methoxypyrimidine formula V, and keep at Stir and react at 0°C to 5°C for 1 hour, when the reaction is over, heat up to 20°C to 30°C, filter, transfer the filtrate to a reaction bottle, evaporate the solvent first, then add 1000g of water, extract three times with 1000g of dichloromethane, The organic layers were combined, the solvent was evaporated, and refined with ethyl acetate to obtain 4-chloro-5,6-dimethoxypyrimidine Formula II 162.5g as a white solid, with a yield of 93.0%.
Embodiment 2
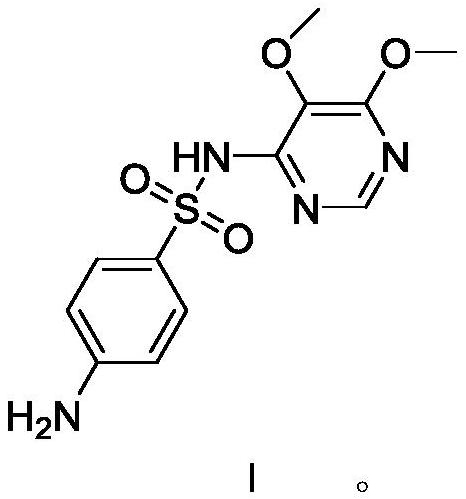
[0033] The preparation of embodiment 2 sulfadoxine formula I (comparative experiment)
[0034] (See State Administration of Medicine. National Compilation of Raw Materials Processes, P172-175)
[0035] Add 200 g of methanol, 90 g (0.466 mol) of 28% sodium methoxide, and 50 g (0.159 mol) of condensate formula VI to the reaction flask in sequence, heat up to reflux for 4 hours, distill off methanol, and add water to distill off aqueous methanol. Use dilute acetic acid to adjust pH=10-11, add activated carbon for decolorization, filter, adjust the filtrate to pH=5.1-5.4 with dilute acetic acid, filter, wash, and dry to obtain sulfadoxin formula I124.5g, yield 80.2%; HPLC purity 98.0 %, of which the impurity of condensate formula VI is 0.6%
Embodiment 3
[0036] The preparation of embodiment 3 sulfadoxine formula I
[0037] Add DMF262g, sulfonamide sodium formula III97.1g (0.50mol), 4-chloro-5,6-dimethoxypyrimidine formula II87.3g (0.50mol), potassium carbonate 103.7g into the reaction flask, after adding Raise the temperature to 80°C-85°C and react for 2 hours. After the reaction, turn on the vacuum, and recover DMF by distillation under reduced pressure. After steaming, add water, stir until dissolved, adjust the pH to 7-8 with dilute acetic acid, cool, filter, and filter the cake Sulfonamide can be recovered.
[0038] Transfer the filtrate to another reaction flask, adjust pH=5.1-5.4 with dilute acetic acid, filter, wash, and dry to obtain sulfadoxine formula I141.5g, yield 91.2%; HPLC purity 99.8%, no condensate formula VI impurities .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com