Method for one-pot synthesis of sulfadoxine by monitoring reaction progress through HPLC
A sulfadoxine and reaction process technology, applied in the field of medicine, can solve the problems of long synthesis period, low yield, no quality control, etc., and achieve the effects of being beneficial to environmental protection, shortening reaction period and reducing waste water discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
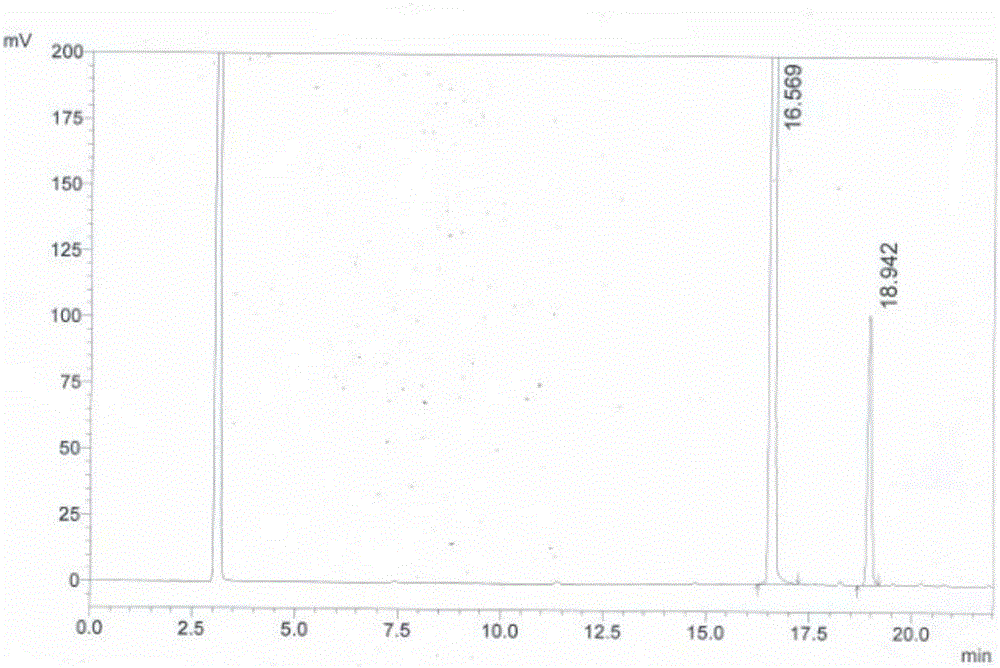
[0054] HPLC system suitability method: Weigh 20mg of 4-sulfonamide-5-methoxy-6-chloropyrimidine and 1.5mg of 4,6-dichloro-5-methoxypyrimidine respectively, add them to a 25mL volumetric flask, and dilute with acetonitrile to Scale, inject 10mL, the peak with a retention time of 16.569min is 4-sulfonyl-5-methoxy-6-chloropyrimidine, the peak with a retention time of 18.942min is 4,6-dichloro-5-methyl Oxypyrimidine, the test results are as follows figure 1 and shown in Table 1.
[0055] Table 1 HPLC system suitability test results
[0056] the peak
keep time
Peak area
Peak area%
Number of theoretical plates
Separation
1
16.569
8514800
92.535
193454.026
0.000
2
18.942
686923
7.465
159273.796
13.937
total
/
9201723
100.00
/
/
[0057] Wherein, the parameter of HPLC instrument is:
[0058] (1) Chromatographic column: C18 column, 4.6mm×150mm, 5μm;
[0059] (2) F...
Embodiment 2
[0063] (1) Preparation of sodium salt: Add 30g of sulfonamide and 60mL of dimethyl sulfoxide to the reaction pot, then add 7g of sodium hydroxide, react at 70°C for 1 hour, add 60mL of benzene, reflux and dehydrate for 2.5 hours, until no water emerges after reflux ≥ After 30 minutes, stop heating and cool to ≤40°C;
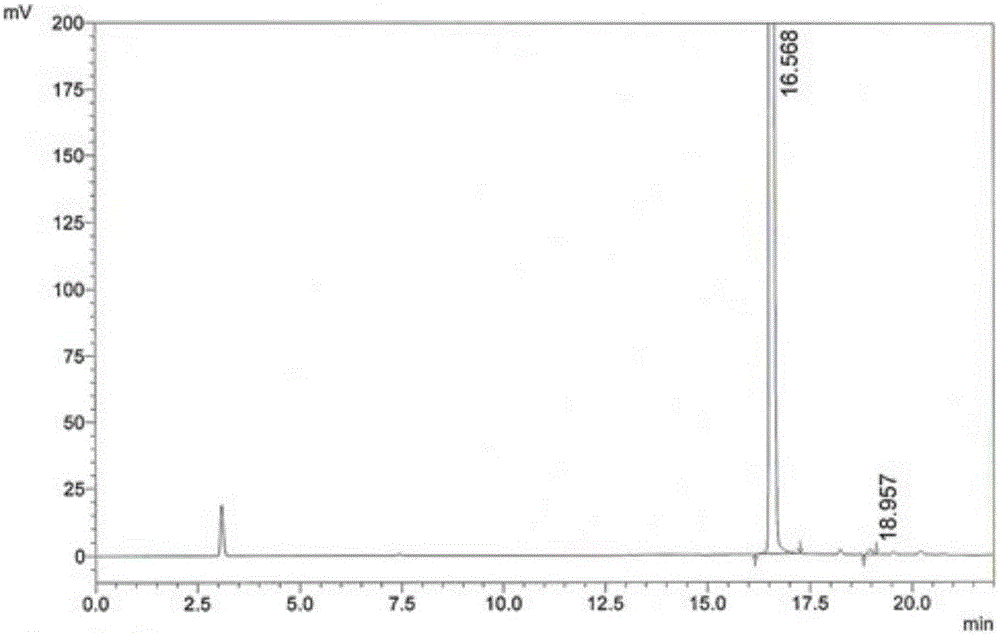
[0064] (2) Condensation reaction: Add 15g of 4,6-dichloro-5-methoxypyrimidine to the reaction pot of step (1), react at 70-90°C for 2-3 hours, distill benzene under reduced pressure, and reach a temperature of 80 ℃, vacuum > 0.08KPa, when there is no solvent flowing out from the outlet of the condenser, stop heating, take a sample, and monitor the residue of 4,6-dichloro-5-methoxypyrimidine in the reaction solution by HPLC: Take 60 μL of the reaction solution to a 25mL volumetric flask , dilute to the mark with water, inject 10 μL, calculate the peak area ratio of 4-sulfa-5-methoxy-6-chloropyrimidine and 4,6-dichloro-5-methoxypyrimidine, and the detection results...
Embodiment 3
[0069] (1) Preparation of sodium salt: Add 30g of sulfonamide and 15mL of water to the reaction pot, then add 7g of sodium hydroxide, react at 70°C for 1 hour, add 90mL of benzene, reflux and dehydrate for 4.5 hours, until the reflux does not produce water for more than 30 minutes, stop Heating and cooling to ≤40°C;
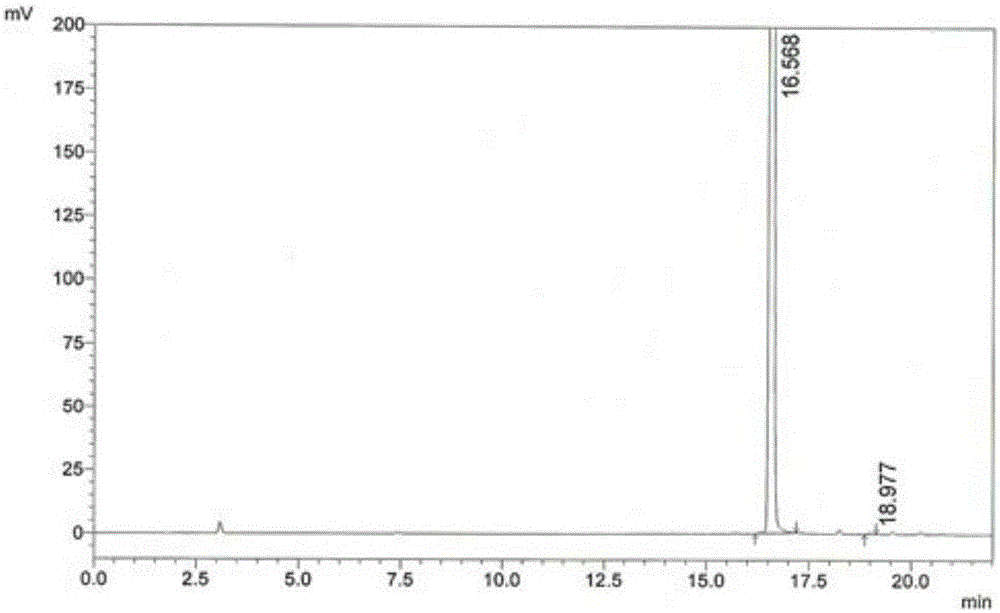
[0070] (2) Condensation reaction: Add 15g of 4,6-dichloro-5-methoxypyrimidine to the reaction pot of step (1), react at 50-80°C for 2-3 hours, distill benzene under reduced pressure, and reach a temperature of 85 ℃, vacuum > 0.08KPa, when there is no solvent flowing out from the outlet of the condenser, stop heating, take a sample, and monitor the residue of 4,6-dichloro-5-methoxypyrimidine in the reaction solution by HPLC: Take 60 μL of the reaction solution to a 25mL volumetric flask , dilute to the mark with water, inject 10 μL, calculate the peak area ratio of 4-sulfa-5-methoxy-6-chloropyrimidine and 4,6-dichloro-5-methoxypyrimidine, 4,6-dichloropyrimidine T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com