Preparation method of high-purity sulfadoxine
A sulfadoxine, high-purity technology, which is applied in the fields of medicine and chemical industry, can solve the problems of inability to reach, achieve the effect of low cost, small amount of solvent, and outstanding substantive characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Sulfadoxine intermediate condensate and finished product HPLC detection method
[0036] Chromatographic conditions:
[0037] Chromatographic column: L11 2.0×10cm, 3μm
[0038] Mobile phase: A: Phosphoric acid aqueous solution (0.1%); B: Acetonitrile (100%) (A:B=83:17)
[0039] Detection wavelength: 230nm
[0040] Flow rate: 0.3ml / min
[0041] Injection volume: 5μl
[0042] Determination method: Take an appropriate amount of this product, accurately weigh 50mg of the sample into a 50ml volumetric flask, first add 5ml of acetonitrile to dissolve, then dilute to the mark with diluent, shake well, and then get it, measure 5μl and inject it into the liquid chromatography, record the chromatogram , calculated according to the peak area normalization method.
Embodiment 2
[0043] The preparation of embodiment 2 sulfadoxine intermediate condensates
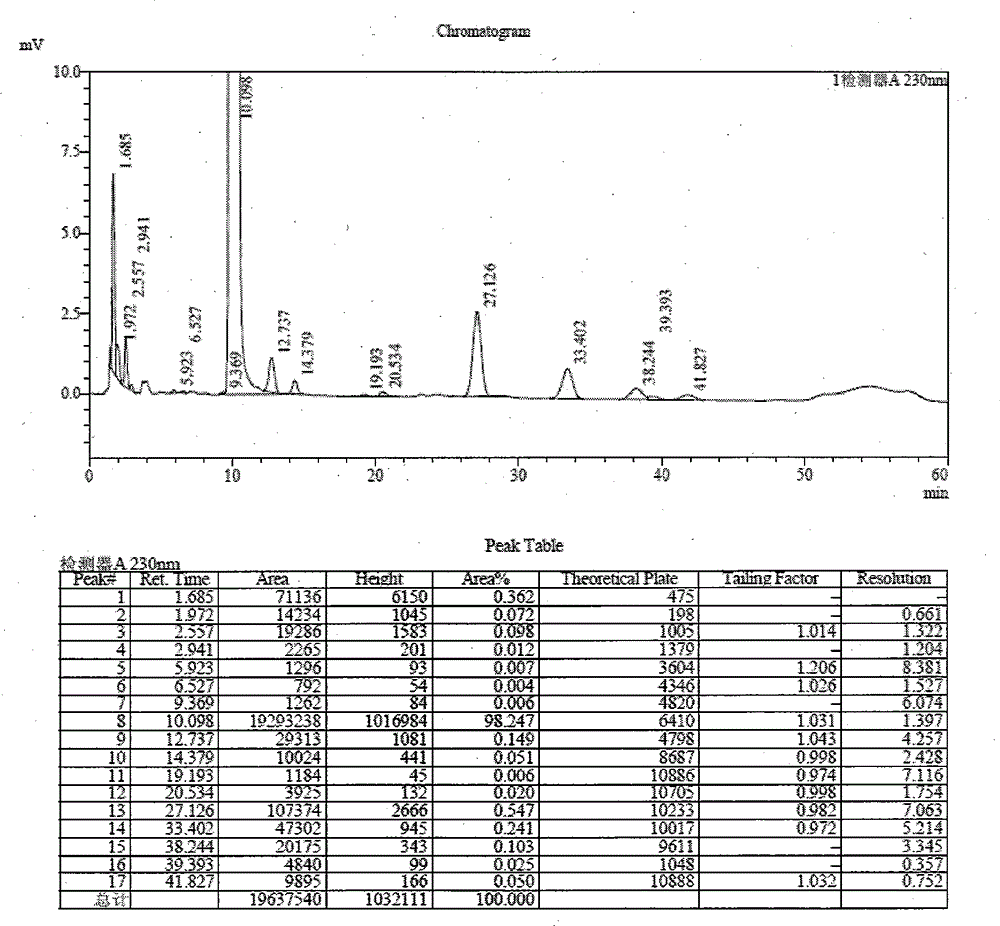
[0044]Add 150g of N,N-dimethylformamide into the reaction flask, add 110g of sodium sulfa, and 50g of 4,6-dichloro-5-methoxypyrimidine under stirring, raise the temperature to 88°C-90°C for 3 hours, reduce Pressurize and recover DMF to a temperature of 120°C, add 200g of hot water above 90°C, dissolve completely, adjust the pH to 7.0-8.0 with dilute hydrochloric acid, cool, filter, and use 5% dilute hydrochloric acid to adjust the pH of the filtrate to 3.8-4.1 to precipitate the condensate. Filtration and drying gave 75 g of sulfadoxine intermediate condensate, with a yield of 85.3%. HPLC total impurity: 1.75%, HPLC maximum single impurity: 0.55%. See attached for details figure 1 .
Embodiment 3
[0045] The purification of embodiment 3 sulfadoxine intermediate condensate
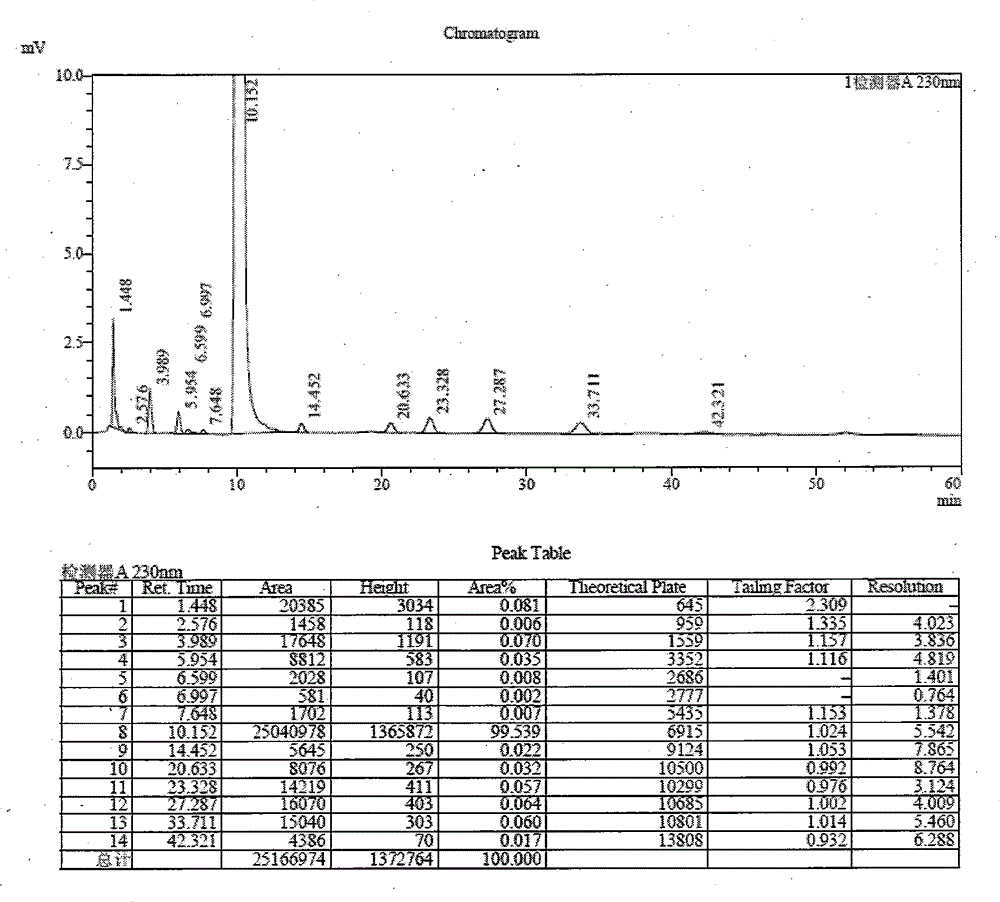
[0046] Add 20 g of N,N-dimethylformamide and 20 g of toluene to the reaction flask, add 20 g of the sulfadoxine intermediate condensate (obtained in Example 2) under stirring, heat until dissolved, cool to 0°C to 10°C and keep warm Stir and crystallize for 55-65 minutes, filter and dry to obtain 13.04 g of off-white powder condensate, yield 65.2%, HPLC total impurity: 0.46%, HPLC maximum single impurity: 0.08%. See attached for details figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com